For five years, I have been doing stem cell research studying the testicles (or testes) of the fruit fly, those tiny ones that you would see in rotting fruit (see Figure 1). And also, for five years, I have been asked by friends and family: “why on Earth would you work with fly testes?”

First, I’ll try to explain why the fruit fly Drosophila melanogaster has been a remarkable model organism in biology for over a century. The first peak of popularity of this insect with bright, red, compound eyes was in 1910, when Thomas Hunt Morgan observed a male fly with white eyes (see Figures 1 and 2). At a time when it was still not clear how characters were transmitted from one generation to the next, and Mendel’s work was being revisited, Morgan wondered if this unusual feature would be present in the following generations of flies. His work ended up confirming the chromosome theory and showing that some inherited characters are sex-linked. Since then, Drosophila has become an extensively used model organism.

Its popularity relies on the fact that many biological processes are conserved between flies and humans, with the advantage that, quite often, these involve a simplified regulation in flies, which makes their study easier. From a technical point of view, Drosophila allows us to control gene expression temporarily in a cell-specific manner, apart from having a wide catalogue of mutants and gene expression reporters available. For over one hundred years, contributions of Drosophila to biology are innumerable, with particular relevance in genetics, development, and neuroscience; among many others.
By now, hopefully you are thinking: “OK, well, I get the fruit fly part. But… Why the testes?’ I’ll try to give an answer to this as well. My research focuses on stem cells, which are a very special subset of cells that can change their identity and differentiate to give rise to different cell types. Besides, they also divide to reproduce themselves and keep providing new differentiated cells when necessary. For instance, human intestinal stem cells give rise to epithelial cells, so the entire intestinal epithelium is renewed in less than a week. Another example are the hematopoietic stem cells, which reside in the bone marrow and can generate all the different cell types present in our blood.
As I mentioned before, numerous biological processes occurring in flies also take place in humans. Flies, as humans, develop from an egg or zygote into an adult organism with a great variety of cell types. Additionally, adult flies and humans generate new cells in their tissues to replenish others lost to damage or natural turnover. The cells responsible for both phenomena are stem cells. Consequently, we could use fruit flies to study stem cell behaviour, and take that knowledge to human biology with the aim to prevent tissue degeneration or aging, among other applications.
Stem cell biology has substantially benefited from the use of Drosophila. In 1978, work with hematopoietic stem cells led Ray Schofield to hypothesise that stem cells would reside in specialised areas within the tissues that support them. He coined these theoretical areas as “niches”. One year later, a structural study based on electron microscopy reported a germinal proliferation centre in Drosophila. A possible stem cell niche in the testes that ensures a continuous production of sperm throughout the life of the fly was observed, but not characterised. Solid evidence that both ovaries and testes contained stem cell niches would not arrive until 1998 and 2001, respectively, being this the reason for using fly testes in stem cell research.
As you might have come to imagine, Drosophila is a really versatile experimental model and the basic mechanisms of how its stem cell niche in the testes works are well known. In this tissue, a cluster of cells anchored to the tip of the testes send previously identified signals to the surrounding stem cells (see Figures 3 and 4). In this way, we can easily design experiments with fly testes to better understand what influences stem cell behaviour. Indeed, fundamental mechanisms of stem cell biology have been identified using this tissue as a model.
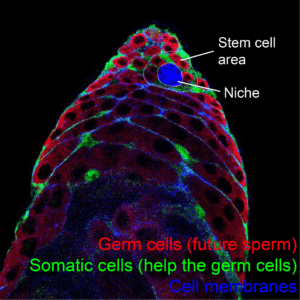
By using fly testes, it has been described that signals that make stem cells divide do not only come from the niche, but also from other stem cells in the tissue, and those signals have been also characterised. Research with Drosophila testes has provided some evidence on how different stem cells coordinate to give rise to their differentiated progeny, so a proper balance of the required cells is met. Work on this tissue has also shown that differentiation of stem cells is not a default process that takes place when signals from the niche are not available, as previously thought. Instead, the change of identity from a stem to a differentiated cell is an active process that requires its own signalling and, once again, the molecules involved have been described. Another lesson we have learned from fly testes is that stem cells are continuously replacing each other: some remain as such close to the niche, while others are lost to differentiation. However, some mutations make stem cells colonise the niche, making them more likely to maintain their position, even displacing other stem cell populations.
During my work as a post-doctoral researcher, we have described for the first time that the communication between the niche and the stem cells is bidirectional. Not only the niche supports the stem cells, but stem cells contribute to the proper functioning of the latter, and we have characterised the molecule involved in this process. We have also found that stem cells that receive signals to divide repress a wide variety of genes involved in a specific metabolic pathway, leading to a state incompatible with differentiation. This state ensures that dividing cells remain as stem cells, and that only when they locate further from the signals coming from the niche they then become competent to differentiate.
I hope I have convinced you about the logic behind using Drosophila testes and how useful model organisms such as this species can be in biological research. It might sound weird first but, after all, we are just trying to find ways to answer elusive questions!
By Diego Sainz de la Maza Redondo, @DSainzdelamaza, post-doctoral research associate at Marc Amoyel’s Lab at the Department of Cell and Developmental Biology, UCL.