Oxygen is something as common as breathing — could not have worded it better! However, oxygen is not as simple as you may think. When talking about oxygen, we are also referring to the third-most abundant element in the universe with regards to mass, just right after hydrogen and helium.
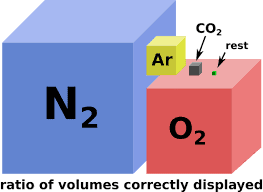
Oxides, compounds containing at least one oxygen atom and one other element, make up almost half of the Earth’s crust [1]. Under standard temperature and pressure, two atoms of oxygen bind to form a molecule of diatomic oxygen. As you have surely read before, the resulting molecule is the famous O2. Although today’s atmosphere is dominated by nitrogen (about 78%), we need to bear in mind that diatomic oxygen currently constitutes approximately 21% of the Earth’s atmosphere. Small amounts of argon, carbon dioxide, and water vapor complete the composition of our current atmosphere. Although everything you have just read may sound quite common to you, things have not always been like that.
It is believed that the atmosphere was strongly reducing, and hence it lacked free oxygen. Its composition was dominated by nitrogen, carbon dioxide, and methane, a completely unbreathable environment for us. Appreciable concentrations of free oxygen were probably not present until about 2,500 million years ago [2]. The presence of large amounts of dissolved and free oxygen could have led to the extinction of most of the organisms that existed at that time. The metabolism of these beings did not depend on oxygen, which is why they are known as anaerobes. For them, in fact, oxygen was a powerful poison. The rise in oxygen levels is attributed to photosynthesis conducted by cyanobacteria, organisms believed to have developed around 3.5 billion years ago. Free oxygen is produced by the light-driven splitting of water during oxygenic photosynthesis. According to some estimates, green algae and cyanobacteria in marine environments provide about 70% of the free oxygen produced on Earth, and the rest is produced by terrestrial plants [3]. In this way, despite the great importance that the Amazon jungle has toward biodiversity and ecology, perhaps the term of it being “the lungs of the planet” should be reconsidered in favour of the oceans.
An overall formula for photosynthesis (6 CO2 + 6 H2O + photons → C6H12O6 + 6 O2) indicates us that photosynthetic organisms manage to synthesize simple sugars (C6H12O6) from carbon dioxide (CO2) and water (H2O) using light energy (i.e., photons) in this process. The oxygen in this formula (O2) is a “waste product” of this process and, as such, it is released by these organisms into the atmosphere. And here the good part begins.

The presence of oxygen in the atmosphere contributed to the development of aerobic respiration, which relies on O2 and is present in all eukaryotes. Therefore, oxygen is also present in higher multicellular organisms such as plants and animals. This metabolic strategy produces a much greater amount of energy than that of anaerobic organisms and made it possible for more complex life forms to be generated. Consequently, these organisms also had a greater energy demand. Another important function of oxygen is achieved when it forms a triad, that is, when three oxygen atoms join together in a molecule. In this case, ozone (O3) is generated, a compound that forms a layer in the troposphere which protects us from ultraviolet radiation from the sun. Otherwise, ultraviolet radiation would reach the Earth’s surface, which will end up harming all the living beings. This is the “good” ozone. Nevertheless, there is also a “bad” ozone, the one that exists “where it shouldn’t exist”: on the earth’s surface (or troposphere). In this case, it is a harmful agent for living beings since it causes numerous respiratory problems.
The presence of oxygen in the atmosphere also led to the appearance of molecules called reactive oxygen species (ROS). The best known of these molecules is the famous hydrogen peroxide (H2O2). ROS are molecules that are naturally present in low concentrations in cells of all types, where they play various physiological roles such as cell signalling, homeostasis, and immunology. However, under certain circumstances, ROS levels can increase beyond physiological levels and, given their high reactive capacity, they can cause damage to all kinds of biomolecules, a process known as “oxidative stress”. This event suggests that ROS has a dual role; whether they act physiologically or as harmful element depends on the balance between ROS production and disposal [4,5]. In other words, oxygen toxicity can arise from both an uncontrolled production of ROS and their inefficient elimination by the antioxidant system.
As we can see, oxygen is an element of great power and value. It is then not surprising that aerobic beings have a sensor system for oxygen levels in our organism, a subject in which I am involved at a professional level and which will be the basis of my next post on the SRUK/CERU Blog — stay tuned!
To wrap up, just let us remember that an adult human at rest inhales about 2 grams of oxygen per minute [6]. In that way, we would say that humanity annually consumes more than 6,000 million tons of oxygen, which will come from nature, photosynthetic beings, plants. Therefore, it is worth reflecting on the fact that, given the circumstances we currently face with regards to the health of our planet, we must hope and wish that photosynthetic beings keep producing all this amount of oxygen, a vital element for all living beings.
By Jose María Cabeza Fernández. Specialist technician at the Institute of Biomedicine of Seville.
More information:
- Atkins P et al. (2016). Chemical Principles, 7th edition. Freeman. ISBN 978-1-4641-8395-9.
- Information via Wikipedia about the Paleoatmosphere, available online here.
- Fenical, W (983). Marine Plants: A Unique and Unexplored Resource. Plants: the potentials for extracting protein, medicines, and other useful chemicals (workshop proceedings). DianePublishing. p. 147. ISBN 978-1-4289-2397-3. Available online here.
- Waszczak, et al. (2018).
- Edreva, A (2005).
- Flow restrictor for measuring respiratory parameters, patent US622456-B1, search performed on google here.