In Chemistry, as in other scientific areas, discoveries are usually named after the researcher who found them. For example, in the daily routine of an Organic Chemistry lab, it is very common to use Swern’s or Stille’s reactions, or Grignard’s reagent without caring much about who invented them. When I find one of these, I like to draw the face and give a voice to the people behind the discoveries. Today I want to remember Sir Robert Robinson, who won the Nobel Prize in 1947 “for his investigations on plant products of biological importance, especially the alkaloids”. By the way, he was nominated for the Nobel a total amount of 51 times in 19 years, between 1928 and 1947.[1,2] Let’s get started.
In Chemistry, as in other scientific areas, discoveries are usually named after the researcher who found them. For example, in the daily routine of an Organic Chemistry lab, it is very common to use Swern’s or Stille’s reactions, or Grignard’s reagent without caring much about who invented them. When I find one of these, I like to draw the face and give a voice to the people behind the discoveries. Today I want to remember Sir Robert Robinson, who won the Nobel Prize in 1947 “for his investigations on plant products of biological importance, especially the alkaloids”. By the way, he was nominated for the Nobel a total amount of 51 times in 19 years, between 1928 and 1947.[1,2] Let’s get started.
On his early years, Robert Robinson was interested in nature, especially Mathematics to which he wanted to dedicate his life. However, his father, an inventor that owned a family business, asked him to study something with a better life perspective: Chemistry. He even built a Chemistry lab for his son at his house! Following this suggestion, Robinson graduated in Chemistry at the University of Manchester in 1905 and started his Ph.D. under the supervision of W. H. Perkin Jr., another well-known chemist of that period. It was then when he became interested in the compounds that would lead him to the Nobel Prize. Since 1910, Sir Robert and his wife, Gertrude Maud Walsh would work as lab partners in universities in cities such as Sidney, St Andrews, London, Oxford or Liverpool. Robinson was already working at the latest, the city of the river Mersey during the WWI. His lab was converted into one of the small “Chemistry colonies” established around Britain those days to develop new dyes and push the English chemical industry. The merge of these colonies gave rise to the British Dyestuffs Corporation, with Robinson as first research director. His students described him as a passionate teacher, a little bit shy and difficult to talk to in a first contact, who didn’t need any notes. He used to mix the syllabus with his own anecdotes. Finally, his passion would drive him to found Tetrahedron, a Chemistry journal that is still running today and to write a textbook that was published after his death. [3]
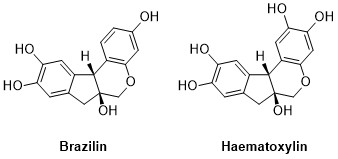
Even if he was awarded the Nobel Prize for his research on natural products, Robinson’s work was widely extended to other areas of Organic Chemistry. For instance, his studies on the structures of natural molecules and his efforts to synthesize them, which would lead him to design a huge number of chemical reactions. At the time, the structure of a molecule was usually discovered by degrading or breaking it into small pieces until already known compounds were found. To verify the reproducibility of the new reaction, these compounds were reacted with one another until the initial molecule was synthesized. These processes, which took several years and are unthinkable now, as some of the modern techniques allow us to elucidate the structure of molecules in as far as a “click”. By breaking and creating, Robinson discovered the brazilin and haematoxylin. These two molecules, extracted from some trees, have been widely used on dyes for their bright red and purple color respectively (Figure 1).
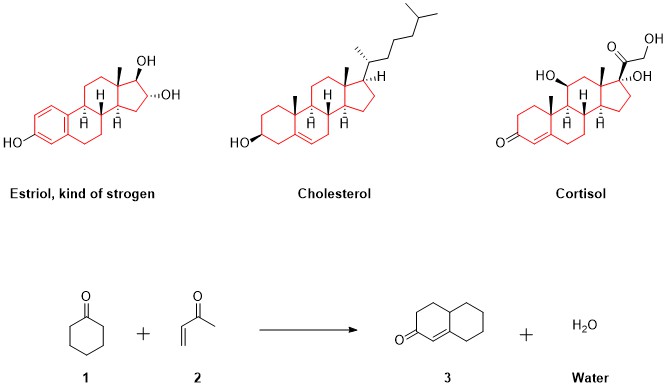
Other natural products he was very keen on studying were steroids. These compounds that we normally associate with sports doping are essential to our life. Some examples of natural steroids are estrogens, cholesterol or cortisol (Figure 2). The main characteristic of steroids is their shared structure. All of them have a main four-ring carbon core (three containing 6 carbon atoms and one containing 5, highlighted in red) to which some chains and atoms are connected. When Robinson tried to synthesize this core, he designed his most famous reaction: Robinson’s annulation. Without getting into detail, by reacting the molecules 1 and 2, he obtained water and molecule 3 which is similar to the bottom rings of the steroids. This can be easy to see when comparing molecule 3 with cortisol (Figure 2).
Robinson’s annulation, described for the first time in 1937,[4] and his more modern versions are regularly used to build new compounds.[5] The formation of new carbon-carbon bonds and, especially the creation of cycles that are usually seen on natural molecules, has been always the Holy Grail of the Organic chemists as they are extremely challenging. Robinson was one of the first reseachers who managed to synthesise them from cheap and accessible reagents as well as in a fast reaction!
To sum up, this is a small snapshot of the work and life of a man who contributed to the development of Organic Chemistry in its early years. His work laid down the foundations of current research projects and his studies on natural products turned to be a pivotal point in the History of Organic Chemistry. Much more could be said about the man or the scientific tools he created.[6] However, we only remember him when we use his most famous reaction or, in my case, when I work at the building named after him in Liverpool.
By Dr. María Pin No KTP Associate at the University of Nottingham and Cornelius Specialties. SRUK Midlands constituency
More information:
References:
1. More than one person can nominate the same awardee on a particular year for the Noble Prize.
2. Sir Robert Robinson-Biographical. (n.d.) Retrieved 20th November 2017 from https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1947/robinson-bio.html
3. Lord Todd; Cornforth, J. W. Robert Robinson, 13 September 1886 – 8 February 1975. Biogr. Mems. Fell. R. Soc. 1976 22, 414.
4. By du Feu, E. C.; McQuillin, F. J.; Robinson, R. Synthesis of substances related to the sterols XIV. A simple synthesis of certain octalones and ketotetrahydrohydrindenes which may be of angle-methyl-substituted type. A theory of the biogenesis of the sterols. Journal of the Chemical Society 1937, 53
5. A)Gallier, F; Martel, A; Dujardin, G. Enantioselective Access to Robinson Annulation Products and Michael Adducts. Angewandte Chemie International Edition. 2017, 56, 12424. B)Linghu, X.; Kennedy-Smith, J.J.; Toste, F. D. Total Synthesis of (+)-Fawcettimine. Angew. Chem. Int. Ed.. 2007, 46, 7671.
6. Zydowsky, T. M. (n.d.). Robert Robinson. Consultado 20 de Noviembre de 2017, en http://www.chemistryexplained.com/Pr-Ro/Robinson-Robert.html