How many times have we heard that nature is wise and that we can learn a lot from it? Hundreds, right? In this blog entry, I bring you another example of this fact with some novelties you might not be aware of!
Sunlight provides us with energy that we can use in form of heat or electricity through solar thermal and photovoltaic panels, among others. So far, we already know this. What if I tell you, however, that this same sunlight can also remove CO2 from, for example, the gas stream of an industrial facility? This sounds newer, right? Or maybe not, because, if we think twice, plants do something similar within the photosynthesis process: they convert water and CO2 present in the air into sugars, alcohols, and oxygen; thanks to the sunlight. Similar to photosynthesis, there is equipment that, in addition to generating storable energy in form of fuels, is capable of sequestering CO2.
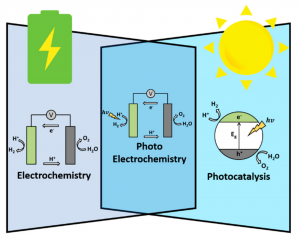
Photoelectrochemistry brings together the three concepts this equipment is based on: photoelectrochemical cells. Photons (sunlight) activate and provide the required energy to move the electrons from specific compounds (CO2, H2O, etc.) located in the cells and combine one (CO2) with another (H2O), which in turn creates new chemical compounds such as methane (CH4). Methane is the main component of natural gas (yes, the same one that we use at home!). In other words, photons from solar light help to initiate chemical reactions with certain compounds (also known as reactants) to generate new products. This process is carried when electrons are transferred from reactants to products and, also, it occurs thanks to other compounds that are part of this process, which are called electrocatalyts. If the reactants are chosen wisely, the desired product can be obtained. Therefore, one of the applications of photoelectrochemical systems is the conversion of CO2 into fuels and valuable chemicals in industry. Some authors call this process artificial photosynthesis or production of solar carbon fuels.
In the current energy and environment context we are living, one of the most important challenges we are facing as a society is to join the use of renewable energy sources, like sun, with the management of the energy demand. The energy generated from renewable sources is characterized by high intermittence and variability, which can result in ‘non-dispatchable’ energy. That means that it is required to have the proper energy storage to use it a posteriori when we really need it. Moreover, this process has to be done under a sustainable management, which means that it has to be clean and avoid the increase of CO2 net emissions.
The systems for the CO2 solar conversion into fuels and chemicals can be classified in three groups: photocatalytic systems (PC), electrochemical systems supplied electrically with photovoltaic cells (PV-EC), and photoelectrochemical systems (PEC). Below, I describe you the main ideas of the latter.
PEC systems mainly consist of two electrodes, an electrolyte and an electrocatalyst. In some cases, there is also a membrane to separate both the anodic and the cathodic cell compartments. One of the electrodes (or even both) is made of semiconductor-photoactive-based material (photoelectrode) and they are named photo-anode and photo-cathode, depending on which one receives the sunlight. There are also cells in which both electrodes can be illuminated. The following figure (Figure 1) shows the diagrams of three-electrode photoelectrochemical cell configurations.
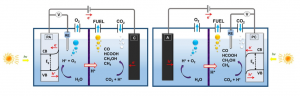
The model that simulates what happens inside the cell considers that the water oxidation reaction occurs in the anode. In this reaction, CO2 is reduced in the cathode to organic compounds, such as methane. To do this, the protons (H+) pass through the membrane separating the two cell chambers. Redox reactions (a term used to describe oxidation and reduction reactions) are:
Anode half-reaction: 2H2O –> O2 + 4e– + 4H+ ; E0 = -1.23 V (E0 vs. NHE at pH=0)
Cathode half-reaction: CO2 + 8H+ + 8e– –> CH4 + 2H2O; E0 = 0.17 V (E0 vs. NHE at pH=0)
These systems are not thermodynamically favourable, that is, they need an external supply of energy to promote the movement of electrons and to achieve that reactions occur in each electrode; which is done by applying an electrical potential (V). It should be noted that the energy needed depends on the amount of electrons to be “mobilized” in the reaction and how stable the molecule is. For example, CO2 is a very stable compound, and therefore more expensive (in terms of energy) than hydrogen. That’s why these “solar fuel” production systems only make sense if they are conceived as a way for storing the surplus of electricity produced by renewable energy. This renewable electricity would be the one that would feed the photoelectrochemical cell and it would convert the CO2 into fuels such as methane, the main component of natural gas.
In case methane is to be obtained as a storable solar fuel, recent publications report faradaic efficiencies up to 67% [2], which means a CO2–to–CH4 conversion rate higher than 80%, although this is not the only carbon-based compound that can be obtained: other high-value products in the industry are formic acid, methanol, ethanol and carbon monoxide [1]. To properly characterize the operation of photoelectrochemical cells, other parameters such as current density (mA/cm2), applied external voltage (V), production ratio (mmol/l.h.cm2), stability (h), and “solar to fuel” efficiency (STF, %), [1] are also analyzed.
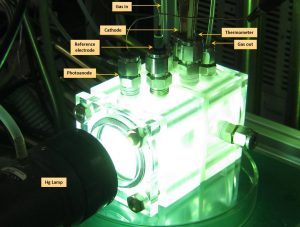
Halmann et al. [3] reported in 1978 the first study of a cell based on a p-type photocathode gallium phosphide which produced methanol, formaldehyde, and formic acid. From then until nowadays, research groups have focused their studies on different cell configurations for photoelectrochemical and photocatalytic systems, as well as on the photoelectrodes materials. Depending on the materials selected for the electrodes and electrolyte and on the energy provided, one product or another can be obtained.
During my stay at the RCCS centre at Heriot-Watt University in Edinburgh, I analysed photoelectrochemical systems (PEC) for methane production and its efficiencies, as well as its similarities and potential synergies with other electrochemical CO2 conversion technologies in which I am carrying out my PhD at the University of León.
The development of this CO2 direct solar reduction technology is currently at a low technological level, that is, at the laboratory level (Figure 2). Research is focusing mainly on finding new photoelectrode materials to be more efficient and abundant in nature. The latter condition is vital for these technologies to be developed under the premises of economic and environmental sustainability and, why not, geostrategy.
By Ruth Diego García, Industrial Engineer at CIUDEN’s Technological Development Center, PhD candidate at University of León.
Thanks to the SRUK/CERU ‘On the Move’ mobility programme and the financial support from my home University (University of León, Department of Environmental Chemical Engineering and Bioprocesses), I was granted a research stay at the RCCS group at the end of 2019, which was supervised by Professor Mercedes Maroto-Valer at the Heriot-Watt University in Edinburgh. The RCSS works on different research lines of CO2 capture, utilisation, and storage systems (CCUS).
More information: