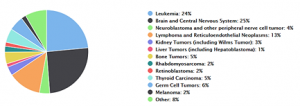
Cancer is well known as an age-related disease, as it is most commonly diagnosed among older people. Nevertheless, according to a study published in The Lancet, nearly 400,000 children and teenagers (0-19 years old) are diagnosed with cancer every year [1]. Then, why is cancer occasionally arising in youngsters?
Unfortunately, there is no clear answer to this question at the moment. The effects of lifestyle-related unhealthy habits have not had time to show up, genetic mutations are not yet accumulated, and there is evidence that only a small percentage of childhood cancers (~10%) are caused by directly-inherited genetic traits [2]. Massive improvements in cancer treatment in childhood have increased the overall survival of these patients from 20-30% (back in the 1960s) to 80-85% nowadays. Mostly, these advances were originated thanks to finding the best combination of classic, already chemotherapeutic drugs for each particular childhood cancer, and dosage optimisation.
However, as nice as it may sound, it is not all a bed of roses: there are subsets of children with advanced, metastatic, or other particularly malignant types of cancer whose survival do not exceed the 30%. For some reason, these tumour types are resistant to the treatments, meaning that cancer cells have the ability to escape the drugs and keep proliferating. In addition, such treatments often cause severe and even life-threatening side effects. In this group of patients, no progress on survival rates has ever been made. Overall, survival for all childhood cancers has not increased over the last few decades, getting stuck at 80-85%. This might indicate that we have already extracted the maximum potential out of the classic chemotherapeutic drugs, and chances are that new chemotherapeutic combinations will not be more effective than any other current treatment in childhood cancer. Consequently, it is crucial to discover new drugs to improve the outcomes in these young patients.
The so-called “molecularly targeted therapies” have revolutionised the field of drug discovery in cancer. Typically, chemotherapy is based on the administration of toxic chemical compounds that act broadly all over the body, predominantly attacking cells that divide rapidly (e.g., cancer cells). This broad activity leads to a certain degree of toxicity and side effects in the patient, as some healthy cells will also be damaged. In contrast, molecularly targeted therapies are biological agents or compounds that block or interfere with “molecular targets”, which are specific cellular elements (e.g., molecules, proteins, pathways, etc.) that are only found in cancer cells and that somehow are involved in the growth or spread of the tumour cells [4]. Thereby, targeted therapies have key advantages if compared to chemotherapeutics: they are much more selective against tumour cells (i.e., therapeutic gain is increased) and side effects are reduced. For that reason, they are considered as the best solution to raise survival chances in these young cancer patients with aggressive tumours.
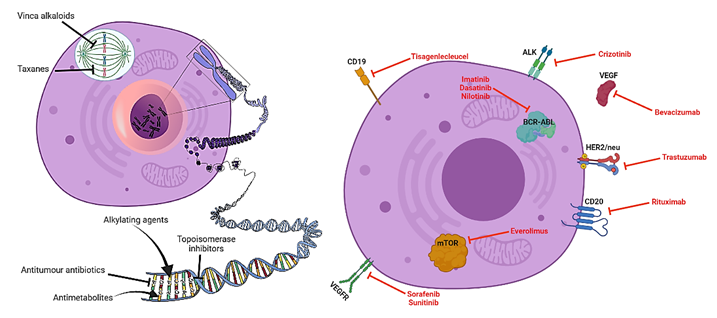
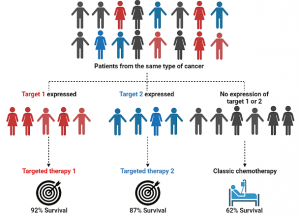
The discovery of targeted therapies stems from the rising in knowledge of the biological and molecular characteristics of tumours. New molecular techniques are allowing the gathering of huge amounts of biological data from tumours and discovering potential targets at an unprecedented pace. In addition, different subtypes from one single cancer type can be described, according to differences in molecular features. This ramification of cancer patients means that tumours now cannot only be labelled with names, but also surnames. Consequently, tumours with the same name but different surname can be treated distinctly with different molecularly targeted drugs. This is the principle of precision or personalised medicine. A very representative example of this is breast cancer (name). This type of cancer is the most prevalent in women and can be classified into different subtypes (surnames) depending on biological characteristics such as HER2+, ER+, BRCA+, Triple Negative, etc. [5]. Several molecularly targeted approaches are available to treat each of these subtypes, and this has dramatically increase the survival of breast cancer patients to a 90% [6].
The topic of my PhD is focused on a type of childhood cancer known as Rhabdomyosarcoma. It remains one of the most deadly childhood cancers, and it mostly arises in the skeletal muscle (i.e., muscles under voluntary control, responsible for our physical movement), although the real origin is not fully understood yet. In our lab, we are trying to develop a type of targeted therapy for Rhabdomyosarcoma, having CYP450 enzymes as targets. This is a very large group of enzymes in our body that carry out plenty of chemical reactions, including the metabolism of drugs. Some studies suggest that particular classes of CYP450 enzymes can be uniquely expressed in Rhabdomyosarcoma tumours [7]. The fact that the target is only found in the tumour tissue is key, as we want to exploit the capacity of these target enzymes to activate prodrugs. In a nutshell, prodrugs are drugs in an inactivated, non-toxic form that become activated into a functional drug after being metabolised by a certain enzyme. Certainly, if the target enzymes were substantially expressed in other healthy tissues, the prodrug would become activated in other areas of the body, and thus resulting in strong side effects. We have developed prodrugs which are specifically activated by the CYP450 isoforms present in Rhabdomyosarcoma. Therefore, our hypothesis is that these CYP450-activated prodrugs could constitute an ideal treatment for this disease. The active drug would only be released into the tumour, and thus would have a much more localised effect while reducing any potential side effects.
Table 1. Current molecularly targeted therapies approved for the treatment of childhood cancers. Each column shows the name of the drug, indications in childhood cancer, and the name of the target that these inhibit.
| Drug | Indications | Target |
| Denosumab | Bone cancer | RANKL |
| Ipilimumab | Colorectal cancer, Melanoma | CTLA-4 |
| Nivolumab | Colorectal cancer | PD-1 |
| Everolimus | Giant cell astrocytoma | m-TOR |
| Pembrolizumab | Hodgkin Lymphoma, Non-Hodgkin Lymphoma, Merkel Cell Carcinoma | PD-1 |
| Blinatumomab | Acute Lymphoblastic Leukaemia | CD19 / CD3 |
| Dasatinib | Acute Lymphoblastic Leukaemia, Chronic Myelogenous Leukaemia | BCR-ABL |
| Imatinib | Acute Lymphoblastic Leukaemia, Chronic Myelogenous Leukaemia | BCR-ABL |
| Tisagenlecleucel | Acute Lymphoblastic Leukaemia | CD19 |
| Gemtuzumab Ozogamicin | Acute Myeloid Leukaemia | CD33 |
| Tagraxofusp | Blastic Plasmacytoid Dendritic Cell Neoplasm | CD123 |
| Nilotinib | Chronic Myelogenous Leukaemia | BCR-ABL |
| Avelumab | Merkel Cell Carcinoma | PD-L1 |
| Dinutuximab | Neuroblastoma | GD2 |
| Naxitamab | Neuroblastoma | GD2 |
| Selumetinib | Neurofibromatosis Type I | MEK1/2 |
| Crizotinib | Non-Hodgkin Lymphoma, Anaplastic Large Cell Lymphoma | ALK |
| Entrectinib | Solid tumours | TrkA/B/C, ALK, ROS1 |
| Larotrectinib sulfate | Solid tumours | Trk |
Despite being quite recent, the incursion of molecularly targeted therapies in the landscape of cancer treatment has already had outstandingly positive effects in adults. Even if some therapies of this type are nowadays used to treat children, the legislations have historically put spokes in the wheels for their testing and approval in these patients. Therefore, progress in treatment for childhood cancer is always lagging behind in comparison to adult cancer therapy. Today, regulatory bodies in the sector are becoming aware of this problem, and are starting to revert to these politics and support the development of further targeted drugs for children. If we, the scientific community, are able to foster the development of these therapies for children over the next years, survival rates are bound to exceptionally rise in these young heroes that are battling cancer. Let’s make this happen!
By Enric Arasanz Picher (@EnricArasanz), PhD Student at University of Bradford.
More information: