Last week I was on a terrace having a coffee with some friends enjoying the sun in Alicante when my daughter got close crying and moaning. Apparently she had fell and hit her knee, experiencing an unpleasant feeling. Although she did not understand what was going on, she decided to sit close to me and wait until the pain was gone. Intuitively, she realized the uncomfortable feeling she was feeling was not quite right, choosing to stop playing and wait for the pain to gradually go away. That is indeed the role of pain, to warn the organism about damage and initiate a protective response on the affected area. Furthermore, it helps us to identify and avoid potentially dangerous situations in the future.
Nociception is the formal term scientists use to describe the physiological processes allowing us to perceive noxious stimulus, thus helping us to survive. There are indeed individuals suffering from a rare condition called congenital insensitivity to pain whose life expectancy is greatly compromised (from 3 to 25 years long), because they are unable to feel pain. Interestingly, although these individuals do not show impairment of their central nervous system function, they have an inherited inability to perceive noxious stimuli. This is because one type of neurons within their peripheral nervous system called primary afferent neurons, are insensitive to them.
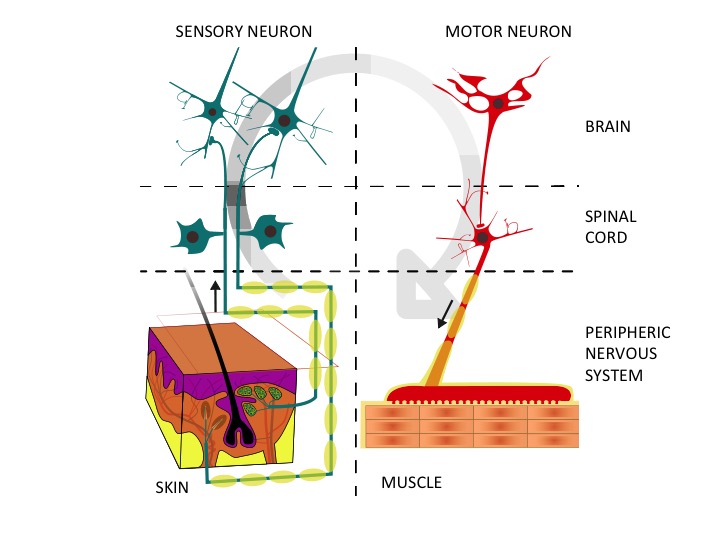 Right: the afferent or sensory neurons collect noxious information from the environment through the nerve endings and bring it to the central nervous system. Left: the motor neurons transport information from the brain to the muscles. Adapted from Wikimedia Commons
Right: the afferent or sensory neurons collect noxious information from the environment through the nerve endings and bring it to the central nervous system. Left: the motor neurons transport information from the brain to the muscles. Adapted from Wikimedia Commons
Human nervous system is the most complex structure in our body, being subdivided in two main parts: the central nervous system composed by the spinal cord and the brain, and the peripheral nervous system, spread throughout our body. The latter can be further divided into sensory neurons, involved in detecting and sending information regarding the environment and autonomic neurons, responsible for sending information about our own body to the brain. The primary afferent neurons are a type of sensory neurons which detect actual and potential tissue damage, sending this information to the central nervous system.
There is yet another rare condition that works on a completely different way when compared to the former. Individuals suffering from inherited erythromelalgia feel constant pain because their primary afferent neurons have an abnormally low activation threshold. In essence, stimuli that would not normally activate these sensory neurons are interpreted as painful, therefore sending nociceptive signals to the central nervous system. It does not come as a surprise these people have a tendency to develop depression and anxiety or even commit suicide, rarely though.
Fortunately, the incidence of both conditions is rather small (being as small as 1 out of 100 million on the first example). However, their study is extremely valuable, as they allow scientists to understand the physiological processes involved in pain detection and conduction to higher brain centres.
 Surprisingly enough, the underlying cause for the impaired pain response in both pathologies relies on the same protein, an ion channel. There are multiple types of proteins expressed throughout our body carrying on different unique roles. Some of them are important from a structural point of view, being scaffolds for other cells or tissues like collagen. Others, like actin and myosin have a mechanical role allowing the cells from our heart to contract pumping blood to every other cell within our body. Ion channels belong to another type of proteins whose main role is to allow specific ions (such as sodium or calcium) to flow either into or out of the cell creating electrical signals that allow cells to communicate among them. Although virtually all cells do express ion channels, in neurons and cardiomyocytes is where they accomplish the most important tasks, as the concerted action of several of these ion channels allow them to encode and send information to distant places in neurons or initiate and propagate the excitation-contraction coupling on cardiomyocytes.
Surprisingly enough, the underlying cause for the impaired pain response in both pathologies relies on the same protein, an ion channel. There are multiple types of proteins expressed throughout our body carrying on different unique roles. Some of them are important from a structural point of view, being scaffolds for other cells or tissues like collagen. Others, like actin and myosin have a mechanical role allowing the cells from our heart to contract pumping blood to every other cell within our body. Ion channels belong to another type of proteins whose main role is to allow specific ions (such as sodium or calcium) to flow either into or out of the cell creating electrical signals that allow cells to communicate among them. Although virtually all cells do express ion channels, in neurons and cardiomyocytes is where they accomplish the most important tasks, as the concerted action of several of these ion channels allow them to encode and send information to distant places in neurons or initiate and propagate the excitation-contraction coupling on cardiomyocytes.
A key ion channel involved in initiating and propagating the electrical signal embodying nociceptive information is the voltage-dependent sodium channel Nav1.7 (encoded by the SCNA9 gene) whose activation leads to the flow of sodium ions into the primary afferent neurons thus depolarizing them. The depolarization allows neurons to communicate and transmit signals throughout the nervous system. Different scientific teams have discovered that, both the chronically painless and painful conditions previously mentioned, are the consequence of mutations in SCNA9 gene. In patients with congenital insensitivity to pain, the identified mutations lead to a truncated form of the protein that is unable to be activated, thus preventing the afferent neurons from activate and therefore sending nociceptive information to the brain. Individuals suffering from inherited erythromelalgia bear mutations on Nav1.7 leading to a gain-of-function phenotype, meaning that the activation threshold for the activation of the protein is greatly reduced. This results in the primary afferent neurons being constantly stimulated and sending misleading nociceptive information to the central nervous system.
It is therefore surprising the finding that the same ion channel can actually behave as a pain switch, either preventing or exacerbating the perception of stimuli that can be neutral or excessively painful. More remarkable for scientists is the fact that Nav1.7 represents a valuable tool for therapeutic intervention, by modulating the activity of the ion channel. When thinking about inherited erythromelalgia, one could envision the development of a specific blocker (often, not an easy task) that attenuates the activity of Nav1.7, thus alleviating the perception of pain. Perhaps more difficult would be to deal with congenital insensitivity to pain, because we would need to express a fully working version of Nav1.7. Approaches would be to either edit the mutated SCNA9 gene from the patient (there is already technology allowing us to do such thing) or to deliver a healthy gene into the primary afferents by using an adenovirus as a delivery tool (the so called, gene therapy).
In summary, studying the underlying causes of a particular disease can provide precious information about how a physiological process works allowing scientists to understand them and ultimately try to cure people suffering from them.
Glossary:
Nociception: the processes of encoding and processing of noxious stimuli by the nervous system.
Primary afferent neurons: first sensory neuron in the body periphery. Their nerve endings encode information related to noxious stimuli, as well as the properties defining them (heat intensity or sharpness of pain, etc). They send information to the central nervous system, while efferent neurons send information to periphery.
Ion channels: membrane proteins forming a channel allowing specific ions to flow across them when open. Ions flow down the electrochemical gradient, thus changing the voltage difference of the cell.
Depolarization: sudden electrical change within a cell decreasing the difference of charge of the neuron. Neurons have a net resting negative membrane potential, which becomes less negative when depolarization is triggered.
Dr. Sergio Laínez Vicente, Senior Research Associate, University of Bristol. SRUK South West Constituency