The invention of the modern refrigerator by Charles Tellier in the 1850s, which was originally based on the compression and expansion of the flammable fluid ammonia (NH3), revolutionised the way we preserve perishable foods, transport medicines and vaccines, and control the temperature in our vehicles, workplaces and homes. However, despite the undeniable success of this technology which nowadays primarily uses hydrofluorocarbons gases that are not flammable, the urgent necessity to replace these environmentally harmful gases along with the necessity to increase the energy efficiency of fridges and air-conditioners, has led to an intense search for alternative cooling technologies that are based on solid materials.
 Amongst the most promising solids for cooling applications, we find the so-called caloric materials. These materials posses the particular ability to change their internal structure when subjected to the application of a stress (such as a magnetic field, an electric field or a mechanical stress), making possible the release or absorption of heat. The physics behind these phase transitions in refrigerant solids is somewhat similar to the physics behind the gas-to-liquid phase transitions in refrigerant fluids that are exploited in current refrigeration systems, which when released to the atmosphere deplete the ozone layer and contribute greatly to global warming.
Amongst the most promising solids for cooling applications, we find the so-called caloric materials. These materials posses the particular ability to change their internal structure when subjected to the application of a stress (such as a magnetic field, an electric field or a mechanical stress), making possible the release or absorption of heat. The physics behind these phase transitions in refrigerant solids is somewhat similar to the physics behind the gas-to-liquid phase transitions in refrigerant fluids that are exploited in current refrigeration systems, which when released to the atmosphere deplete the ozone layer and contribute greatly to global warming.
The best example of caloric material is perhaps an ordinary party balloon, which is made of rubber. In its normal state, a party balloon consists of a network of disordered polymer chains, which somewhat resemble a plate of spaghetti. On stretching the balloon, the polymer chains align in the direction of the applied force, increasing the degree of internal ordering of the chains, and decreasing quantity that in the physical sciences is known as entropy, which represents the degree of order of a substance. If the stretching is done very fast, this change in internal structure leads to an increase in temperature that can be easily detected using your lips (please do try this at home!), or in a more sophisticated manner using an infra-red camera. On releasing the balloon after it has recovered its initial temperature, the balloon gets cold, and it is at this stage that it can be used for cooling another object.
 Caloric materials driven by magnetic or electric field have been intensively developed in research laboratories around the world during the last twenty years. However, their commercial application still seems far from being accomplished due to the following severe impediments: large magnetic fields are challenging to generate economically, and electrocaloric effects are only large in very thin materials. With the aim of overcoming these difficulties, research community is gradually focusing on shifting toward caloric materials that are operated by mechanical stress (particularly by hydrostatic pressure). Yet, until very recently there was only a very small number of good barocaloric magnetic materials (as these hydrostatic-driven materials are named), the majority of which are based on non‑abundant expensive chemical elements. But this trend is now rapidly changing.
Caloric materials driven by magnetic or electric field have been intensively developed in research laboratories around the world during the last twenty years. However, their commercial application still seems far from being accomplished due to the following severe impediments: large magnetic fields are challenging to generate economically, and electrocaloric effects are only large in very thin materials. With the aim of overcoming these difficulties, research community is gradually focusing on shifting toward caloric materials that are operated by mechanical stress (particularly by hydrostatic pressure). Yet, until very recently there was only a very small number of good barocaloric magnetic materials (as these hydrostatic-driven materials are named), the majority of which are based on non‑abundant expensive chemical elements. But this trend is now rapidly changing.
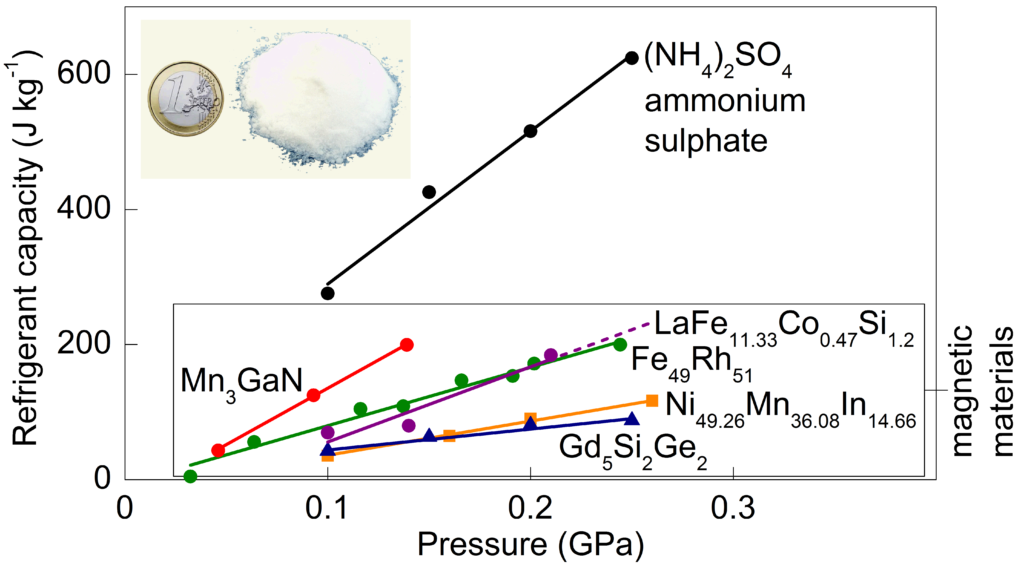
This figure represents the cooling capacity of the best barocaloric materials existing nowadays, relative to the atmospheric pressure applied (0.1 GPa = 1000 atmospheres).
My research group at the University of Cambridge is now developing novel barocaloric materials based on abundant inexpensive chemical elements! Our first breakthrough arose in salts of ammonium sulphate [(NH4)2SO4], a cheap material (€0.06 per gram) highly abundant in nature, and traditionally used as fertiliser for our crops. The refrigerant response of this material (represented below as refrigerant capacity – which is a measure of how good a material is for cooling) improves upon existing expensive barocaloric magnetic materials, and it opens up an exciting promising era for the discovery of new solids for environment-friendly refrigeration and air-conditioning technologies.
By Dr Xavier Moya, Royal Society University Research Fellow, Department of Materials Science & Metallurgy, University of Cambridge. SRUK Cambridge constituency.