The world report of the World Health Organization about tuberculosis, which is known since the 17th century as The White Plague, announced in 2017 that 1.27 million people died because of this disease and 10 million new cases appeared. In 2016, tuberculosis was the tenth leading cause of death worldwide – it was the sixth in 2000, so it seems we might have done at least something well! Nevertheless, we should be wondering why an infectious disease can still be responsible for so many deaths in the 21st century, mainly in low-income countries.
Children resting in a center for patients with tuberculosis. These places used to be located in isolated regions with a favorable climate so the patients could feel more relaxed and calm, which could help with an easier recovery.
First, the treatment of this disease is complex: the initial stage lasts at least 6 months and requires not less than four antibiotics – and this only applies if the bacterium that causes tuberculosis, Mycobacterium tuberculosis, responds well to the treatment. Note that Mycobacterium tuberculosis is a slow-growing bacterium: it takes nothing less than 42 days to grow! This means that we need to wait 42 days if we want to have enough bacteria to carry out biological tests, such as those testing for antibiotic resistance, which could help us identify the specific bacterial strain that is infecting the patient.
Antibiotic resistance has become a worldwide health problem. The number of antibiotic-resistant bacteria just keeps growing in number, and some of them have even become multidrug-resistant bacteria, i.e., they tolerate all antibiotics known to date. Some of the most well-known multidrug-resistant bacteria are Staphylococcus aureus (staph skin infections, pneumonia, endocarditis, etc.), Pseudomonas aeruginosa (pneumonia), and Mycobacterium tuberculosis itself. How do these antibiotic-resistant bacteria arise? Essentially, this can be due to both the misuse of antibiotics and the quick mutation rate of bacteria.
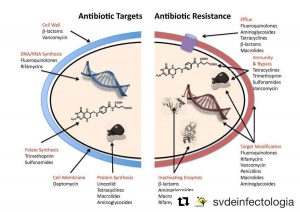
Left: targets that attack the antibiotics. Right: Mechanisms that bacteria have to fight to become resistant to each antibiotic.
For instance, if you had a bacterial infection and the the doctor gave you a pack of antibiotics that you should take as part of your treatment, you would need to TAKE ALL the antibiotics in the pack as prescribed, regardless you start feeling better! Why is that? The truth is that, even though you feel better, it is likely that pathogenic bacteria are still living in your body. These “survivors” are bacteria that have shown a greater tolerance, which is not the same as resistance, to the antibiotic you have been taking. If you make the unwise decision of stopping the treatment before you should, these bacteria will remain in your body, multiply, and eventually cause a new infection. Besides, if this happened, the antibiotic you first took would either no longer work or you would even need to take a much larger dose for it to be effective. We should remember that drugs may have side effects if taken at high doses, hence it is preferable to avoid this scenario. On the contrary, if someone takes the antibiotics for a longer period of time than the one prescribed, the continued presence of this antibiotic will end up triggering the survival mechanisms of the bacteria in their quest for survival. For instance, if a random mutation that allowed the bacteria to survive was favoured by natural selection and hence fixed, these bacteria and their descendants would end up shaping all the bacterial population responsible for the infection, thus being resistant to the antibiotic. Therefore, prolonging the intake of antibiotics longer than needed is as bad as stopping the prescribed treatment earlier.
Accordingly, one could then wonder why new antibiotics are not being produced. Bacteria cannot be resistant to something that they have never seen before, right? Well yes, they indeed can! We must remember that antibiotics are naturally produced by other bacteria, and it is relatively easy to find environmental bacteria or fungi that produce antimicrobial substances. Unfortunately, most of the times the quantity they produce is not enough to generate a drug that could effectively kill the bacteria responsible for the disease. In addition, most of these antibiotics have no commercial interest. Developing new antibiotics poses a very long and expensive process in the pharmaceutical industry, whose long-term investment would not be guaranteed because bacteria can become resistant relatively quick. Therefore, this results in a low antibiotics production, hence to a fewer availability of several types of antibiotics to defeat specific bacteria. Besides, according to recent studies, it is expected that, in 2030, more people will die from microbial infections than from cancer.
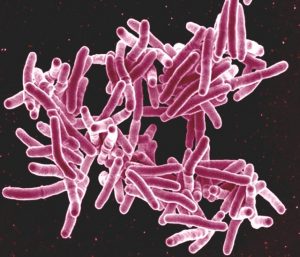
Electron microscopy of the M. tuberculosis bacillus, the bacterium causing tuberculosis.
In the case of tuberculosis, an early detection of antibiotic-resistance is crucial. There are some commercial kits that can directly take a sputum sample, i.e., a biological sample taken from the patient, and test for resistance to the antibiotic rifampicin. Nevertheless, even if bacteria were susceptible to rifampicin, they could also be resistant to any of the other three antibiotics that are given in the initial stage of the treatment. As pointed out above, Mycobacterium tuberculosis is a slow-growing bacterium, thus it is not after several weeks have passed by since the treatment started that one can confirm whether bacteria are antibiotic-resistant. Therefore, in the meantime, the patient is just taking antibiotics that, not only do not work, but could even worsen the the patient’s health by promoting new resistance to other antibiotics.
Currently, several research groups, including ours, are trying to extract, directly from the sputum, a sufficient amount of bacterial DNA to carry out specific techniques of molecular biology that could efficiently identify the lineage of the bacterial strain, as well as its resistance to antibiotics, that has infected the patient. Even though this process is more expensive than the one currently followed, mainly because of the usage of genomic sequencing techniques, its effectiveness leads to more successful treatments and, more importantly, the patients can feel better and recover from the disease much quicker. Only for the latter, the investment on research to further develop these new molecular techniques is more than worth it.
* * *
By Jessica Comín Polo, PhD student at the Genética de Micobacterias research group, based at the Instituto Aragonés de Ciencias de la Salud.
More information: