How many times has humanity dreamt of a universal cure for cancer? According to the World Health Organisation (WHO), cancer is the second leading cause of mortality, only overcome by cardiovascular diseases and accounted for around 10 million deaths worldwide in 2020 [1]. Nowadays, to a greater or lesser extent, we might all know some acquaintance, relative, or friend suffering from this condition, which causes distress to both patients and loved ones around them. Therefore, a total cure for cancer is little less than the Holy Grail of human medicine. Given the key biological and molecular differences between types of tumours, however, it is extremely unlikely that something like this can get to exist. Instead, what we can do now is to try to identify vulnerabilities shared between a wide range of tumours and exploit them in our favour.
One of the most investigated biological pathways related to tumoral responses has been the one involving the Myc protein. This protein operates as the central coordinator of the proliferation of the cells in our body and has been found at exceptionally high levels in around 70% of all cancers [2]. Within the cell, the function of Myc is dependent on its physical binding to its partner, the Max protein, which results in the formation of what we call a protein dimer: the Myc-Max dimer. When this interaction happens, the Myc-Max dimer can be internalised in the nucleus of the cell (where the DNA is found), location where the dimer can then bind to certain regions of the genome called E-boxes. This interaction will subsequently trigger the activation of genes that will execute the cellular division (mitosis). In other words, the combined roles of Myc and Max as a dimer dictate when the cells are ready to divide, and hence order the cellular machinery to initiate this process. Max is structurally similar to Myc except for the active domain that Myc has, and thus cannot activate gene expression on its own.
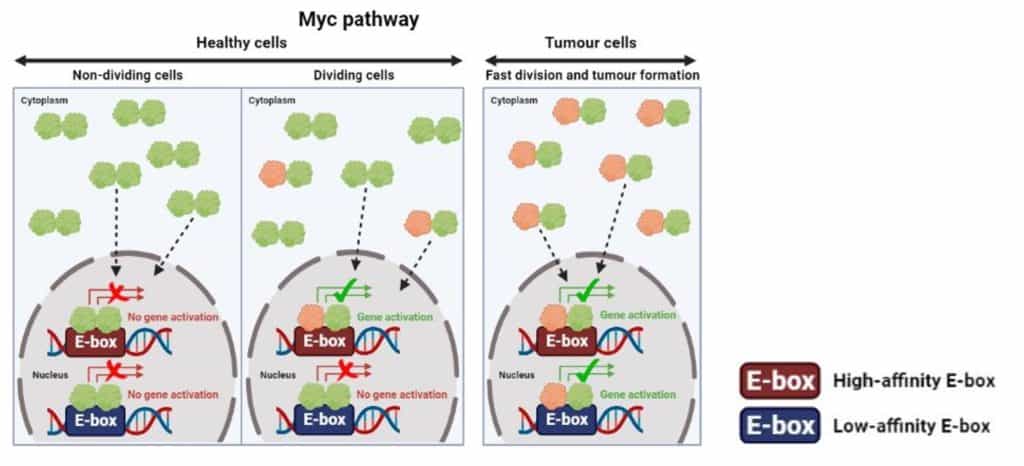
In physiological conditions (healthy state), the abundance of Myc in the cell is tightly regulated to be kept at rather low levels. In that context, the Max protein binds to itself to form inactive Max-Max dimers, which are predominant. Consequently, only a small and transitory number of Myc-Max dimers are available to effectively bind to the E-boxes present in the genome, which is enough for the proper functioning of the cell. However, Myc is abnormally upregulated in patients with cancer, and so the proportion of Myc-Max dimers versus Max-Max dimers increases. This situation results in more Myc-Max dimers binding to the genome, which will end up triggering a cascade of events that include critical changes in cell behaviour, many of them contributing to promote tumour growth such as increased cell proliferation, a higher risk to form metastasis, resistance to cancer therapies, transport of nutrients to the tumour, protecting the tumour against the attack of the immune system, etc. [3].
Consequently, many researchers over the last decades have focused their efforts on the search for an approach with which the Myc protein could be inhibited, aiming to counteract tumour formation or its progression. In essence, this research has made Myc become the ‘most wanted’ target protein in cancer therapy. Nevertheless, due to certain characteristics of the Myc protein, there has always been a general belief about this protein not being able to be targeted. This conception has to do with multiple reasons: the severe side effects that inhibiting Myc in healthy tissues can cause; the lack of pockets in its structure to which drugs could easily bind as part of a molecular treatment; or the intrinsic difficulties in targeting a protein that acts in the nucleus of the cells, and hence hindering the access of most drugs due to the heavily compartmentalisation in the nucleus [4].
Among the researchers trying to debunk this myth, we find Dr Laura Soucek, the Principal Investigator of the Models of Cancer Therapies research group at the VHIO research centre in Barcelona. She is also an ICREA Research Professor and co-founder and CEO of Peptomyc S.L. who is leading a research group that aims to develop a Myc inhibitor named Omomyc [5], which could potentially be the first drug of its type to cross this barrier if the results of the first clinical trials are positive.
Omomyc is a mini-protein that was initially developed in 1998 so it could mimic the Myc protein, but with additional features. To achieve that, Omomyc was developed by simulating one fraction of the Myc protein while including a few modifications to enhance its capacity to bind to other proteins [6], but like Max, it lacks the active domain. While the Myc protein cannot form Myc-Myc dimers, Omomyc can bind to itself, to the Max protein, and to the Myc protein, thereby forming the corresponding dimers in cells: Omomyc-Omomyc, Omomyc-Max, and Omomyc-Myc. Two of these Omomyc dimers, Omomyc-Omomyc and Omomyc-Max, can effectively stop the activation of pro-tumoural genes and suppress the growth of tumoural cells, which will consequently die. On the other hand, the Omomyc-Myc dimer will result in the Myc protein being eliminated [7].
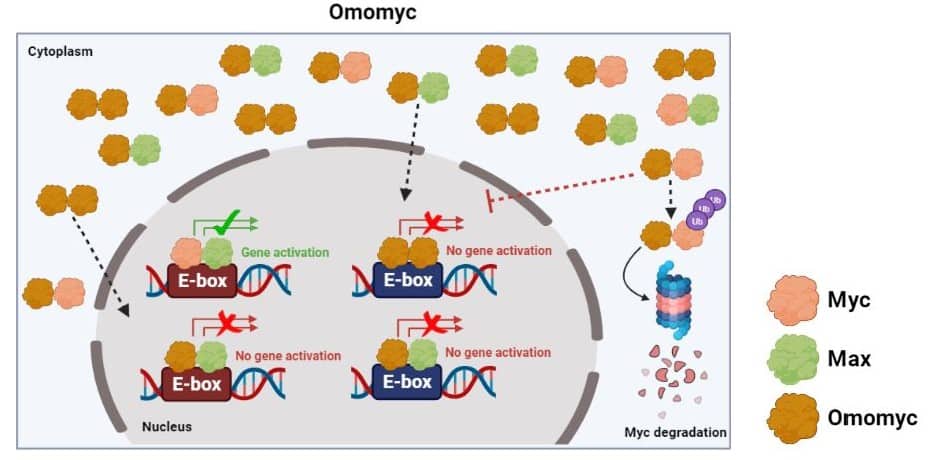
The preclinical efficacy of Omomyc is encouraging given that it suggests a correct uptake by cells and a high efficiency in multiple animal models, both when tackling primary tumours (tumours firstly originated) and metastases [9, 10]. Nowadays, the multicentre Phase I-II clinical trial to evaluate the safety of Omomyc treatment (OMO-103) is underway in Spain, taking place in Barcelona (Hospital Universitari Vall d’Hebron) and Madrid (Hospital Universitario H.M Sanchinarro and Hospital Universitario Fundación Jiménez Díaz). Advanced-stage cancer patients who are unresponsive to other therapies have been enrolled to this trial, which include lung cancer, breast cancer, and colorectal cancer patients [11]. The next steps will involve the follow-up of these patients with regards to the performance of this drug, and whether the cancer they suffer from progresses into more advanced stages of the clinical research.
Nevertheless, even if some of the patients treated with Omomyc managed to fully recover from the disease, this would not mean that Omomyc would become a universal cure for cancer. In fact, chances are that there is never going to be such a thing. The advances in molecular biology within the last decades have led to a greater understanding of cancer biology, as well as of the molecules, proteins, or cellular pathways that are relevant for tumour growth. We can now use this powerful knowledge to design drugs that counteract or target these tumoral signals, such as the case of Omomyc presented here or the case of Trastuzumab (also known as Herceptin), an approved drug for HER2+ treatment, metastatic, and non-metastatic gastric and breast cancers. These are just two examples out of the many biosimilar drugs that have been synthesised in the last years with related applications [12].
Breakthroughs such as Omomyc illustrate the potential that the next generation of drugs can have to treat cancer, which have been developed thanks to the extensive collection and study of tumoral samples for the development of targeted approaches. Omomyc is not only a glimmer of hope for current and future cancer patients, but also for the many researchers working on different ways to inhibit Myc with the main purpose of treating cancer.
By Enric Arasanz Picher (@EnricArasanz), PhD student at the University of Bradford (Institute of Cancer Therapeutics).
More information:
- Cancer. Source: Wolrd Health Organisation (WHO), 2022. Available online here.
- Dang CV, 2012.
- Whitfield JR and Soucek L, 2021.
- Massó-Vallés D and Soucek L, 2020.
- Soucek L et al., 2002.
- Soucek L et al., 1998.
- Jung LA et al., 2017.
- Demma MJ et al., 2019.
- Beaulieu ME et al., 2019.
- Massó-Vallés D et al., 2022.
- Phase 1/2 Study to Evaluate Safety, PK and Efficacy of the MYC-Inhibitor OMO-103 in Solid Tumours (MYCure). Source: ClinicalTrials.gov. Available online here.
- Sawyers CL, 2019.