The intestine is one of the most important organs of the defense system in our body. Are you surprised? Well, it shouldn’t surprise you. The gastrointestinal tract harbours more than 1014 microorganisms (10 times more bacterial cells than the number of human cells), which are collectively known as the intestinal microbiota. Even though every individual has a different microbiota (our intestinal fingerprint), these microorganisms are usually beneficial. Recently, several investigations have described that pathogens and microbial dysbiosis could be important factors that not only are involved in the development of gastrointestinal pathologies, but also in other diseases. Thus, it seems essential to control the “neighbourhood” that lives in our intestine. Do you want to know how this population is controlled? How beneficial bacteria are favourably selected versus pathogens? Mucins have most of the answers to these questions.
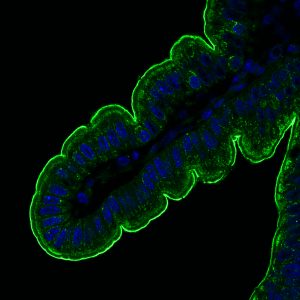
Membrane mucin stained in green in intestinal epithelial cells.
Mucins are large proteins that we can find not only in the intestinal mucus, but also in mucus from the lungs and the genital tract. These mucins create a net to stop anything that can potentially be harmful or damage our organism. There are two types of mucins: those secreted to the intestinal lumen and those anchored to the membrane of cells. On the one hand, secreted mucins are main components of the intestinal mucus in the lumen, where microbiota are trapped. On the other hand, membrane mucins build an additional barrier on the cells that protect the intestine. For instance, imagine that you are a castle where the water in the moat would be the mucus (secreted mucins) and the castle wall would be the membrane mucins.
Intestinal mucus protects the intestine from physical disturbances, overpopulation of microorganisms, and the appearance of pathogens [1]. Nevertheless, this mechanism does not always work properly. In some intestinal pathologies, such as inflammatory bowel diseases and cancer, the properties of the mucus are depleted: there is almost no mucus and it is very permeable. The disruption of the mucus lets the bacteria over-proliferate, which can promote inflammation and provoke damage in the intestinal epithelium. When the protective mucus layer collapses, the second group of mucins show up: membrane mucins. Can you remember them? They have been unnoticed until now, but they are our “castle wall”, probably the most important protective barrier to avoid the proliferation of the disease. Membrane mucins cover the surface of intestinal cells next to the microbiota, in the intestinal epithelium. These mucins build a solid wall all along the intestine that prevents bacterial invasion, defending our castle. The biological function of membrane mucins in the intestinal mucus, however, is poorly understood if we compare it with our knowledge about secreted mucins.
In healthy individuals, bacteria can cross mucus and reach the intestinal epithelium, where they interact with membrane mucins that could trigger intestinal responses [2]. Therefore, membrane mucins may act both as a physical protection and as a bacterial signal transducer to the intestine, bridging the communication between our microbiota and us. An example of how this mucin defence system works is the protection from the intestinal pathogen Helicobacter pylori (H. pylori), which induces stomach ulcers and cancer. H. pylori disrupts the mucus and reaches the intestinal epithelium, where it interacts with a membrane mucin called MUC1. When the pathogen binds to MUC1, one part of MUC1 breaks off and sends signals to the intestine, triggering a protective response against H. pylori. Moreover, the pathogen attached to the shedded part of MUC1 returns to the mucus, where it will be destroyed. Thus, both secreted and membrane mucins prevent pathogen invasion and trigger defensive responses in the intestine at the same time.
In intestinal bowel diseases and cancer, an alteration in membrane mucins that could promote the development of the disease has been noticed. Recent work in intestinal bowel diseases has found mutations in mucins that modify their structure, leading to intestinal barrier dysfunction and bacterial invasion, thus increasing the ongoing inflammation. In cancer, mucins are not only highly expressed, but also their location is aberrant: they are placed in the basolateral membrane instead of the apical surface. This change of location produces a weaker connection of these cells to the intestinal epithelium, which lets them travel to other organs and resulting in metastasis [3].
Research about the intestinal microbiota relationship is at its peak. The design of new pharmacological therapies to influence the microbiota has been proposed to prevent gastrointestinal pathologies and regulate immunological responses in the intestine. Moreover, it seems that, once we better understand this mechanism, we could modulate the interaction between the host and the microbiota in other pathologies, such as obesity, depression, or Alzheimer’s disease; where bacteria have been recently described as an important factor.
In this relationship between microbiota and intestine, membrane mucins are crucial since they are at the intersection between microbiota and the host. Therefore, our investigation about the role of membrane mucins is both timely and needed. In fact, during the last few years, our research group led by Thaher Pelaseyed has received up to 8 research projects funded by different associations, e.g., the Swedish Society for Medical Research (SSMF), National Institutes of Health/Mucosal Immunology Studies Team (NIH/MIST), and the Wenner-Gren Foundations. The main aim of these projects is to describe the relationship between intestine and microbiota, as well as the implication of this interaction in health and disease. In our studies, we have discovered that membrane mucins seem to be a key factor in host-microbiota interactions and have a crucial role in the integrity of our intestinal barrier.
* * *
By Elena Layunta Hernández (@ELayunta), Postdoctoral Researcher at the Institute of Biomedicine, University of Gothenburg.
More information: