DNA failures can cause malfunction of the cell and emergence of diseases. The development of tools in biomedicine, such as gene therapy, which it seeks to restore the damaged cell function, it has open up hope for finding effective treatments to fight against diseases that we considered incurables some years ago.
Deoxyribonucleic acid, DNA, is the genetic material inherited from our parents. Everything happens at the cellular level has to go, somehow, through our DNA. Therefore, we could consider it as our cell boss. But, how does this process happen? To address this question we first have to know that DNA is a chain whose links are molecules called nucleotides, formed by a sugar and a compound called nitrogenous base. In DNA, there are up to four different types of these nitrogenous bases: adenine (A), guanine (G), cytosine (C) and thymine (T). The sequential order in which they are arranged along the DNA chain determines a specific sequence since proteins are synthetized. These proteins are the ultimate responsible for carrying out important cellular processes such as their growth, metabolism or proliferation.
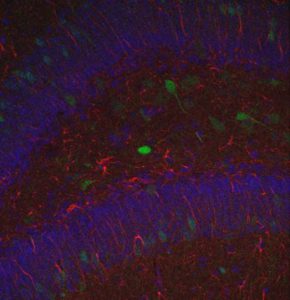
Mouse hippocampus where green cells have been infected with a viral vector that synthesizes GFP (Green Fluorescent Protein), red cells are astorocytes stained for GFAP protein (Glial Fibrillary Acidic Protein) and the celular nuclei are stained in blue by DAPI (fluorescent marker that binds strongly to the nuclear DNA).
But DNA, like all bosses, is not perfect, and it may contain errors in its sequence. In the case of DNA, those mistakes are changes in its nucleotide sequence, called mutations. That is, insertion or deletion of nucleotides, exchange of a single nucleotide for another… The final result is the synthesis of a different protein from the one it should have been generated or simply it does not exist, so that its cellular function is affected. Sometimes these mutations can be beneficial for the specie and/or organism, and, precisely, the Darwin´s theory of evolution is based on that, but, in other occasions they are prejudicial leading to diseases. Biomedical research has made huge progress in the treatment of diseases since beginning on the last century, however, it was not until the 80s-90s when the focus was on trying to supply the mutated gene using an approach called gene therapy.
What is this therapy based on? The idea is very simple to understand but more difficult to carry out: we try to change the gene affected with the correct version of it. Specifically, a short version of DNA without the mutation is introduced into the cells, so that this “new DNA” is capable of producing the right protein, as would the non-mutated DNA. This new protein will substitute the function of the damaged one, restoring the original defect.
One of the limiting factors in this therapy is how to introduce that correct DNA strain into our cells. It is needed a vehicle to carry it, which it is called vector. The development of vector, which combines both efficiency and safety, is key for the success of gene therapy approaches. In many laboratories, such as ours, viral vectors are used. But, why do we use viruses if, usually, virus are linked to diseases? Firstly, viruses are able to infect our cells very efficiently and use the machinery of the host cell for their own benefit. Moreover, due to the current knowledge of the cell biology of many viruses, it is possible to engineer them to produce attenuated virus, ie, without the harmful elements for the cells. So that, they can continue infecting them and using their machinery without causing any damage which it guarantees biosecurity. And, precisely, these attenuated viruses are further modified in the laboratory to carry our new DNA sequence into the cells.
There are numerous examples of biomedical research in gene therapy. Two of them are amyotrophic lateral sclerosis (ALS), the disease became world-wide famous a few years ago thanks to “ice bucket challenge”, and Batten disease. Both are neurodegenerative disorders that can cause the death of the patient a few years after their onset. In the case of ALS, in the “Sheffield Institute for translational Neuroscience”, we use gene therapy approaches to generate ALS-like mice models (Herranz-Martin et al., 2017; Walker et al., 2017), which provide new data for its study, such as the relationship between neurodegeneration and failures in the repairment of damaged DNA (Walker et al., 2017).
My current research at “University College London” is focused on the development of gene therapy for the treatment of Batten disease. This is a rare disease whose onset is usually during childhood or youth. It is mainly characterised by cognitive and motor impairments, visual failures and seizures episodes. Even though there are several variants, it is an ideal candidate for gene therapy approaches because of its monogenic origin, that is, the mutation of just one gene in our DNA is responsible for each variant of the disease, which it makes more direct their study and easier the use of animal models for their research and treatment.
Although there is still a long way to go in the gene therapy field, there are already some product approved for their use, such as Glybera®, wihch it is used for the treatment of acute pancreatitis caused by deficiency in the enzyme lipoprotein lipase. Moreover, there are numerous clinical trials on-going for the treatment of different disorders that use as main approach gene therapy (http://www.wiley.com/legacy/wileychi/genmed/clinical/). Therefore, this technique may help us to find solutions in the future for the disease that were considered incurable a few years ago. Well, gene therapy and other technologies based on genetic engineering as well, such as the famous CRISPR, but I will leave that for another blogger.
By Dr Saúl Herranz-Martín, Postdoctoral researcher, University College London. SRUK London constituency.
References:
- Herranz-Martin S*, Chandran J*, Lewis K, Mulcahy PJ, Higginbottom A, Walker C, Martinez Peña y Valenzuela I, Jones R, Coldicott I, Iannitti T, Akaaboune M, El-Khamisy SF, Gillingwater T, Shaw PJ, Azzouz M. ““Viral delivery of C9orf72 hexanucleotide repeat expansions in mice leads to repeat-length-dependent neuropathology and behavioural deficits”. *Joint 1st authors. Disease Models and Mechansims. 2017 Jul 1;10(7):859-868
- Walker C*, Herranz-Martin S*, Karyka E, Liao C, Lewis K, Elsayed W, Lukashchuk V, Chiang S-C, Ray S, Mulcahy PJ, Jurga M, Tsagakis I, Ianitti T, Chandran J, Coldicott I, De Vos KJ, Hassan M K, Higginbottom A, Shaw, PJ, Hautbergue GM, Azzouz M, El-Khamisy SF. “C9orf72 expansion disrupts ATM-mediated chromosomal break repair”. *Joint 1st authors. Nature Neuroscience. 2017. Sep;20(9):1225-1235.
- (http://www.wiley.com/legacy/wileychi/genmed/clinical/)