In recent years there have been major changes in the aspirations and activities of women. More and more women are looking to consolidate their professional career and achieve some job stability before having children. This, together with the easier access to planned parenthood methods, has resulted in a rise of the average age of women giving birth in Europe, as you can see in the graph below (fig.1) [1]. The problem that lies in this social change is, basically, a greater difficulty for conception. In other words, parenthood is getting more and more complicated to reach for us.
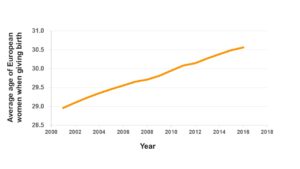
Figure 1. The graph shows how the average age at which European women give birth has increased over the last years.
As we get older, the chances of pregnancy decrease. Women are born with around one million oocytes, which are the cells that will transform into ovules. This number is reduced before puberty, to around 400,000. This is what we called the ovarian reserve. Apparently it is very likely that, at least, one out of near half a million oocytes forms a baby, right? But of them, only 1% becomes a mature ovule, ready to be fertilized. On top of this, the quality of the ovules is progressively decreasing. Therefore, after 35 years, the reproductive potential decreases significantly, and after 40 years the possibility of pregnancy is between 10 and 30%, according to experts [2].
Do not panic. Thanks to assisted reproduction technologies we can have biological offspring and working life without the need for reincarnation through a technique called ovule cryopreservation. In other words, a woman who plans to be a mother after age 35 can freeze her own eggs until she decides to use them. But what happens if you do not have things so clear at 25? What if the desire to be a mother comes by surprise? Or if the infertility comes at a very early age, maybe due to treatments such as chemotherapy or premature ovarian failure? In these cases, the alternative could be trying to use donated eggs. Egg donation is not a simple procedure, as requires a previous treatment with hormones and a small surgical process (called follicular puncture) to collect the ovum just when it leaves the ovary. This supposes, at least, an annoyance to the woman. This is the reason why ovule donors are not as easy to find as sperm donors. As you can imagine, this is an important limitation in assisted reproduction. Fortunately, science might find a solution. There are, at least, two branches of study that are worth a mention in this post. Let’s look into them, step by step.
Imagine that you only had to go to the doctor, provide a sample of saliva with cheek cells and ask them to transform these cells into ovules. Easy, right? No waiting list, no pain, no hormonal treatments. Simply, they would treat those skin cells in such a way that they transform. It may sound like science fiction, but it is not. It is called cell reprogramming and it has already been done in human cells [3]. Cell reprogramming involves the transformation of a cell that is already specialized in another different cell type. To do this, you must first make the cells forget what type of cells they are or “de-differentiate” them. This is currently possible using a set of proteins that control the expression of the genes that are key for the process. These dedifferentiated cells can be transformed into many different types of cells. To specifically re-differentiate them in oocytes, the lab should provide conditions similar to those that allow the formation of oocytes in the ovary (fig. 2a).
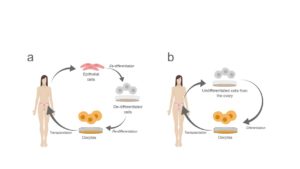
Figure 2. An explanatory diagram of the potential applications of (a) cell reprogramming and (b) in vitro oocyte maturation in reproductive medicine.
The other alternative relates to the ovarian reserve that we mentioned above. Do you remember that it was formed by about 400,000 ovules that were sequentially used in each menstruation? A few years ago, we thought that this reserve could not be regenerated: a woman had a fixed number of egg precursor cells. At the same time, we knew that blood cells naturally regenerate in the human body. In addition, our body contains the so-called stem cells that can gain the characteristics of specialized cells, essentially as if they were already de-differentiated. Well, more recently, it has been discovered that there are also stem cells in the mouse ovary and that, under proper in vitro conditions they can differentiate and become oocytes (fig. 2b). These can be transplanted back to the ovary of a mouse and, in fact, healthy mice have already been born from female mice that were sterile before transplantation. The good news is that this type of cell has also been found in humans [4]!
Truth is that all this is a bit far from the current techniques that doctors can offer us nowadays in an assisted reproduction clinic. Just as we cannot buy flying cars – if they offer you one, I would not trust them. There are still many questions in the air about how cells differentiate (and de-differentiate), how this knowledge can be applied to human reproduction, what effects these techniques might have in the offspring generated, and what the cost of these treatments would be. In addition, it is necessary to carry out an in-depth ethical analysis that deals with the controversy that this new field may generate, because we are not discussing the best way to cook Spanish omelettes, we are talking about people.
What is clear is that science advances by leaps and bounds and that it could break the current limits of reproductive medicine. After all, remember this is to make easier for families to adapt to the new role of women in society.
References
[1] Eurostat
[2] Instituto Valenciano de la Infertilidad
[3] Takahashi, K. & Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 126, 663–676 (2006).
[4] Carroll, J., Whittingham, D. G., Wood, M. J., Telfer, E., & Gosden, R. G. Extra-ovarian production of mature viable mouse oocytes from frozen primary follicles. Journal of Reproduction and Fertility, 90(1), 321-327 (1990).
By Ana I Rodríguez Rodríguez. PhD Student, Wellcome Trust Centre for Cell Biology. University of Edinburgh. Scotland SRUK Constituency.