If my friend Laura had been born in Ancient Greece, she would have been an alchemist. I picture her wrapped in white fabric, casting spells out loud while mixing viscous liquids, trying to find the elixir of life or to create gold. Alchemy began then, or perhaps many centuries before, in Mesopotamia, and lasted until about the XVIII century.
Newton, who was born in the mid 17th century, did not have any problems in combining the study of universal gravitation and mechanical laws with the search for the philosopher’s stone that would transform low-grade metals into gold and silver. 150 years later, though, Dalton formulated his atomic theory, and the few spell books that remained open were closed forever.
Dalton said that matter is made of atoms, just as the pieces of a puzzle create an image, and he described what these atoms looked like. From this model others were developed, until the one we have today which is a distant great-grandson of Dalton’s.
To simplify, we could say that atoms are formed by subatomic particles: protons, neutrons and electrons, which are distributed between the nucleus (protons and neutrons) and its surroundings (electrons). Protons have a positive charge, neutrons have no charge and electrons have a negative charge.
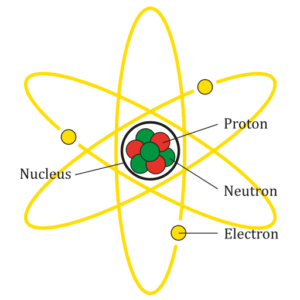
AG Caesar (2018) Atom Diagram.svg [Image] from https://commons.wikimedia.org/wiki/File:Atom_Diagram.svg
In general, an atom has the same number of protons, neutrons and electrons – oxygen, for example, has 8 protons, 8 neutrons and 8 electrons – but this can change. If the number of electrons is changed, we have an
ionof the element. Gaining electrons, we have a negatively charged ion, and losing them, a positively charged one. O
-2 is an oxygen atom with 8 protons, 8 neutrons and 10 electrons. Ions are very reactive because they seek to interact with those with the opposite signs to balance. If the number of protons is modified, what simply happens is the atom becomes another element. MAGIC! It is not that gold’s protons, electrons or neutrons are different from mercury’s – they are identical! The only difference is in the number of protons they have: 79 in gold, 80 in mercury. Alchemists of the world, if you want to get gold, what you should do is steal a proton from the mercury atom. Finally, if what is changed is the number of neutrons, then we have a different
isotope of the element. Uranium-235, for example, instead of having 92 protons and 146 neutrons, as the case would typically be in uranium, has 92 protons and 143 neutrons (92 + 143 = 235, hence the name). Since neutrons have no charge, isotopes are neither positive nor negative, but because they suffer an imbalance between the number of neutrons and protons, they are sometimes unstable. The unstable isotopes release particles and energy to become stable atoms in a process called ‘decay’. The resultant atom can be an atom of another element or a stable isotope of the same element. The energy and particles released are known by the name of
radioactivity. Henri Becquerel was studying uranium salts when one day, by accident, he placed them in a drawer, on top of photographic paper. There was no source of natural light inside the drawer, but the photographic paper developed anyway. He realized the salts were emitting rays by themselves, and let Marie and Pierre Curie continue investigating it. In 1903 the three of them won the Nobel Prize in Physics for discovering radioactivity.

The U.S. National Archives (1948 – 1957) [Image] from https://picryl.com/media/atomic-scientists-969a17
Nuclear power plants use this phenomenon to obtain energy. They hit protons with a mixture of uranium-235 and uranium-238 at high-impact, and artificially cause the breakdown of uranium-235. It releases a large amount of heat that, through turbines and generators, is transformed into electricity. They use a mixture of uranium-235 and uranium-238, and not pure uranium-235, because in the natural world they are mixed-up. However, through various processes called ‘uranium enrichment’, the proportion of uranium-235 can be increased. The final mixture usually does not have more than 5% of uranium-235, leaving a huge amount of unreacted uranium-238 after the whole process. This uranium is a residue and will be stored for good. The PhD project that Laura is doing at the University of Edinburgh is focused on trying to find a use for this uranium-238. Mainly, seeing if it could be used to facilitate the transformation of carbon monoxide and carbon dioxide into other substances, and reducing the concentration of these polluting gases in the atmosphere. It would be a very useful philosopher’s stone for the planet.
Post by Roser Bastida. Communications and outreach technician at Institut de Neurociències de la UAB (Barcelona). SRUK Friend.