In the last 40 years, cancer survival has doubled thanks to improvements in cancer detection, therapy and supportive care [1,2]. In fact, approximately 50% patients diagnosed with cancer survive for 10 years or more, and this survival rate keeps increasing per year [3]. But what happens after you survive cancer?
One major concern is fertility preservation: cancer therapies can be highly toxic to the ovaries, and the treatments can lead to reproductive problems, such as infertility and premature ovarian insufficiency (when ovaries stop functioning normally before 40 years old).
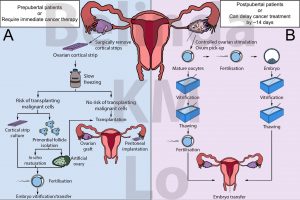
Figure 1. Options for fertility preservation for (A) prepubertal women or those who require immediate cancer therapy; or (B) post pubertal women or those who can delay cancer therapy by approximately 14 days. Adapted from Donnez and Dolmans (2017).
For adult women who may be able to delay cancer therapy, egg or embryo freezing may be considered (Figure 1B). This involves controlled hormonal stimulation of the ovaries to produce eggs. These eggs can then be retrieved for direct freezing, or fertilised with sperm and the resulting embryo can be frozen and stored. After cancer treatment, the eggs can be thawed and fertilised to generate embryos. In the case of frozen embryos, they can be directly transferred back to the patient. Since 2012, egg freezing has been accepted as a fertility treatment by the American Society of Reproductive Medicine [4]. Several clinical trials have also proven that pregnancy rates from frozen eggs are comparable to using fresh eggs [5,6].
However, egg and embryo freezing may not be possible for all patients, such as those with hormone-sensitive cancers, those who require immediate cancer treatment, or prepubertal patients who do not respond to ovarian stimulation (Figure 1A). With more prepubertal patients surviving until childbearing age [1], options to ensure their future fertility must be considered before they undergo cancer treatment.
Ovarian tissue cryopreservation offers a method to preserve the fertility of these patients without the need for hormonal stimulation. The aim of ovarian tissue cryopreservation is to freeze strips of the ovarian cortex (just below the surface of the ovary) which contains a large number of primordial follicles, the basic unit of fertility within an ovary. Each primordial follicle has the potential to grow and generate an egg.
After cancer treatment, these frozen pieces of ovarian tissue can be thawed and transplanted back into the patient to re-establish ovarian function. Some women experience the return of ovarian function for up to 10 years [7]. In fact, it is estimated that 130 children worldwide have been already conceived as a result of ovarian tissue transplantation [8,9,10].
Despite the benefits of ovarian tissue cryopreservation and transplantation, it is estimated that the majority of the follicles are lost during the procedure, specifically when the transplanted tissue redevelops its blood and oxygen supply. In addition, transplantation of ovarian tissue after cancer therapy may also lead to the reintroduction of cancerous cells. This is a concern particularly for cancers with a high risk of ovarian metastasis (e.g., leukaemia, neuroblastoma, Burkitt’s lymphoma) [11].
The risk for cancer reintroduction may be bypassed by developing fertilisable eggs from artificial ovaries. An artificial ovary is small tissue containing a mixture of ovarian cells created to simulate a physiological ovary. These artificial ovaries may also include a scaffold to physically support the cells in a 3D structure, as well as provide additional factors to improve the artificial ovary function. Once generated, these artificial ovaries may be transplanted or cultured in vitro to develop fertilisable eggs (Figure 1A).
Using tissues from cancer patients, various scientific groups are demonstrating the potential applications of artificial ovaries. Follicles have been isolated from ovarian tissues of leukaemic patients with no detectable cancerous cells present [12]. The supporting ovarian cells, which provide factors to support follicle growth, can potentially be isolated from the same patient’s ovaries after cancer treatment, to avoid cancer reintroduction [13].
The generation of artificial ovaries is still experimental, but follicle survival has been demonstrated in a human artificial ovary which was transplanted into a mouse for three weeks [14]. Further work may even look into ovarian stem cells and induced pluripotent stem cells (a type of stem cell generated from mature cells which may be able to develop into any type of human cell needed) as a source of germ cells, particularly for patients who may not have enough follicles in their ovaries. Using adult-derived induced pluripotent cells in mice, a Japanese group were able to generate fertilisable eggs in vitro, which resulted in the birth of eleven healthy pups.
Although the focus here is on cancer patients, the technologies mentioned may also be applicable to other women who require fertility preservation, and to restore ovarian function beyond fertility, i.e., menopause.
* * *
By Dr. Belinda Lo (@belinda_lo), embryologist at the Hong Kong Sanatorium & Hospital. DPhil in Obstetrics and Gynaecology from the University of Oxford.
More information:
- Cancer Research UK. Cancer Statistics for the UK, 2015.
- Ferlay, J. et al. (2015).
- Guzzinati, S. et al. (2018).
- Practice Committee of the American Society for Reproductive Medicine (2012).
- Cobo, A. et al. (2010).
- Rienzi, L. et al. (2010).
- Andersen, C. Y et al. (2012).
- Donnez, J. & Dolmans, M. M. (2017).
- Gellert, S. E. et al. (2018).
- Jensen, A. K. et al. (2017).
- Dolmans, M. M. et al. (2013).
- Soares, M. et al. (2017).
- Andersen, C.Y. et al. (2019).
- Pors, S. E. et al. (2019).
Featured image:
Putting ovarian tissue strips into the preserving solution, Dr. Vereczkey Attila. Source: Wikimedia.