As Led Zeppelin said in 1969,
“Heartbreaker, your time has come. Can't take your evil way. Go away heartbreaker”
As you know, we cannot go by poetry to carry out our research. However, since many of us would have followed the way of the muses had they chosen us, we try to add an artistic touch to our work. By chance, one year after this song was published, a protocol to make the first magnetic resonance imaging device available was approved. Godfrey Newbold Hounsfield based his work partly on that protocol to create the first tomograph, which earned him the Nobel Prize in Physiology in 1979 [1]. The importance of this prize lain in what we call multidisciplinary teams today, which made an electronic engineer get his hands on a medicine-based prize. This way, 50 years later, I have completed the cycle, using knowledge introduced by Hounsfield to try to explain anatomical and physiological changes that our cardiac system undergoes against that feared evil heartbreaker.
If, as a quick mental game, you ask another person to think about a heart, the image that will come to their head will surely be that of an emoji. What they don’t know is that that image is probably that of an hypertrophied heart, that is, a heart showing an abnormal growth, usually associated with diseases like diabetes or obesity. Through the collaboration of my original research group (IP-Dr. Andrés Hueva) and the group that took me in to do my SRUK/CERU’s “On the move” program stay during 2020 (IP-Dr. Pablo Lamata), we managed to link previous knowledge in molecular biology (Laboratory-Dr. Antonio Andrés) with the broad uses of cardiac imaging (Laboratory-Dr. Pablo Lamata), searching for a way to solve this phenomenon.
Previous work carried out at the laboratory made us think about the role of leptin as a possible inducer of changes in the heart. Leptin [2], a hormone produced in our adipose tissues, is able to move through the blood and get to the hypothalamus. There, it interacts with specific receptors for it, activating the sympathetic nervous system, which transmits information to peripherical organs such as the heart, the liver or adipose tissue.
After studying the network of metabolic and inflammatory pathways (i.e. chemical reactions which allow to use and consume energy and essential metabolites for the cell’s functioning, and activation routes of the inflammatory immune response following a stimulus, respectively) following a central and chronic treatment with leptin [3], we reached the conclusion that those seemed to be interconnected, so our treatment was making that emoji-looking image to decrease in size. But how could we be sure that was real? Here is where SRUK and its “On the move” program come in. Without thinking twice, I contacted Dr. Pablo Lamata’s group in the School of Biomedical Engineering and Imaging Sciences (King’s College London). I had read their work on predictive models in silico (i.e. computational) for cardiac hypertrophy and, although it was a little out of my way, I thought that multidisciplinary teams were created for a reason (again, Hounsfield and multidisciplinary research). This way, we could go from that aforementioned mental image to the following one:
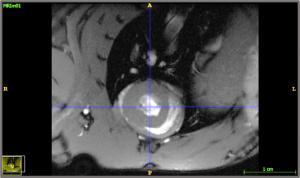
Figure 1. NMR (nuclear magnetic resonance) image in DICOM format (Digital Imaging and Communication On Medicine), showing the two cardiac ventricles. Photo credit: Blanca Rubio.
This image is simply an NMR that uses a powerful magnetic field, radio waves and a computer to produce detailed images of different internal body structures. In this case, we have an image created by plotting a plane perpendicular to the heart’s base, so that the organ appears as a ring. This kind of cuts allow us to study morphological changes associated to our treatment, as well as different physiological parameters. All in all, it gives us a way to interpret our model, in our case, concluding that central leptin, in those doses and times established for our study, does not promote an excessive or abnormal growth of the heart’s walls, which we call hypertrophy.
Finally, we found it necessary to contribute something else related to leptin’s effect on the heart. We decided to create an in silico model of our rats’ hearts to study this organ’s behaviour under various computational models that use different algorithms. This way, once we find the computational model that best explains the behaviour of the heart under the effect of leptin, we will be able to use it to predict the development of cardiac hypertrophy more exactly and accurately. Ideally, these predictions could corroborate previous data obtained experimentally, which would in turn validate (or not) our initial hypothesis: that leptin, at the central level, promotes atrophy (the concept opposite hypertrophy) in the heart.
Unfortunately, time frames in science differ greatly from what is expected, so when we say “we decided”, what it actually means is that I hope to be able to tell you about it in a not too far off instalment of this blog.
* * *
By Blanca María Rubio Muñoz., Dr. in Health Sciences. Research Associate at the Faculty of Environmental Sciences and Biochemistry at University of Castilla-La Mancha (Spain).
More information: