According to the United Nations, the world population is expected to reach 9.8 billion people by 2050 and 11.2 billion in 2100 [1]. It is predicted that this increased population will demand 70% more food than what we already consume today.
The numbers above take into account several factors, such as the changes in dietary habits. Due to higher income levels in developing countries, people will be eating more animal proteins and dairy products, and hence will have to produce more food to feed animals under intensive-feeding systems. In addition, a significant portion of grain is used for biofuel production instead of food consumption [2], which diverts farmland usage in a debate known as “food vs. fuel”. The reality is that most available arable land is already in use. Therefore, the future of agriculture faces the challenge of providing the expanded population with a sustainable and secure supply of food by minimising both the land and the chemical inputs [3].
To date, nitrogen is one of the primary limiting nutrients for plant growth and crop productivity. Nitrogen is an essential element for all living organisms for being the basis of important biomolecules, such as DNA and proteins. Despite being the most abundant gas in the atmosphere (~78%), neither animals nor plants can “breathe” nitrogen. The reason behind this fact lies in the strength of the triple bond joining the two atoms of nitrogen that constitutes atmospheric nitrogen (N2). Due to the consequent difficulty to “break” this triple bond (and hence incorporate it easily into our systems), we all need to add protein in our diet as a source of nitrogen. Before it can be utilised by most living systems, however, the intake protein will be processed and modified it into other chemical forms such as ammonium (NH3) or nitrate (NO4). In the case of plants, this external supply of nitrogen is made through fertilisers.
In Europe, fertilisers have been applied to crops since the early 19th century. The commercial use started in 1802, when the German explorer Alexander von Humboldt and the French botanist Aimé Bonpland brought from their South American expedition samples of ‘Guano‘, a nitrogen-rich fertiliser formed from bird droppings accumulating on the coastal islands of Peru for hundreds of years [4]. These deposits became so valuable that led to a war between Peru and Chile from 1879 to 1883 (War of the Pacific). By the end of the 19th century, the Guano deposits were already depleted, which led again to shortages of nitrogen sources for agricultural use.

In 1909, the Haber-Bosch process, considered one of the most important inventions of the twentieth century, revolutionised the world. This process was developed by Fritz Haber, a German physical chemist known as the father of modern agriculture but also of the modern chemical warfare [5]. By applying the Haber-Bosch approach, the triple bond problem between the two nitrogen atoms was cracked: the use of iron catalysts at high temperatures and elevated pressures could break the bond and generate ammonia. For this discovery, which made the manufacture of ammonia economically feasible, Fritz Haber was awarded the Nobel Prize for Chemistry in 1918. The method was scaled up by Carl Bosch, an industrial chemist who also won a Nobel Prize in 1931. Thanks to both scientists, chemical fertilisers have been used extensively in agriculture since then – indeed, they are also used in our own back gardens! Due to their impact on our daily life, the Haber-Bosch process is known as the “detonador of the population explosion” [6].
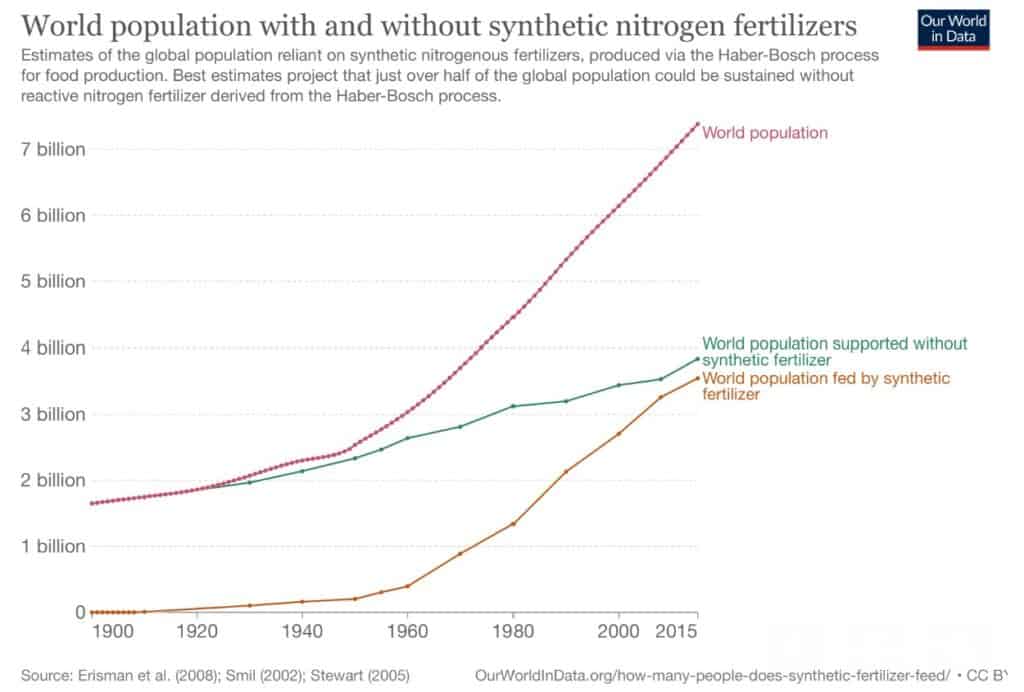
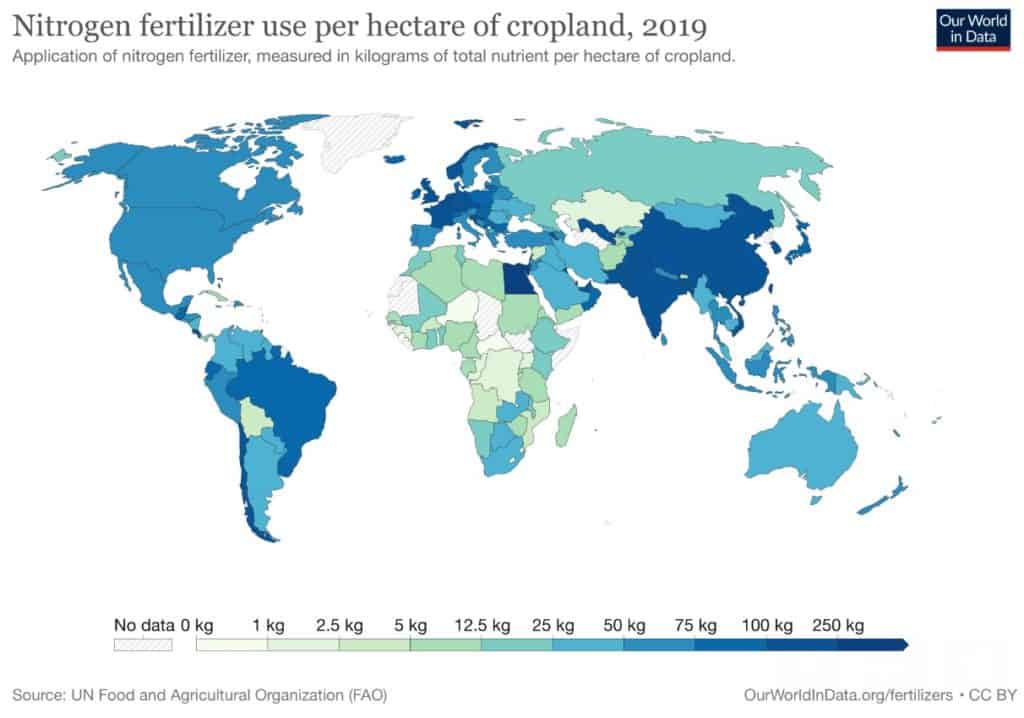
The use of chemical fertilisers is driving the world’s population from 1,600 billion in 1900 to almost 8,000 billion today. Already in 2004, it sustained roughly 2 out of 5 people. As of 2015, it sustained nearly half the people on the planet [7]. As you can imagine, the nitrogen paradox is a complex one: while too little nitrogen is a problem, nitrogen in excess is also a problem: “feast or famine” [8]. In many regions, like sub-Saharan Africa, yields of cereal crops are limited by the economic inaccessibility of the farmers to such fertilisers. Indeed, the recent high input costs, supply disruptions, and trade restrictions are fuelling a severe fertiliser shortage and rising prices worldwide: the World Bank’s fertiliser price index rose nearly 15 percent from earlier this year, with prices more than triple of those in 2020 [9]. On the other side of the story, fertiliser overuse leads to adverse environmental and health impacts such as polluting waterways by leaching from the soil and releasing greenhouse gases. To face these environmental issues, the EU Commission’s Green Deal ‘Farm-to-Fork’ and ‘Biodiversity’ strategies aim to reduce nutrient losses to the environment from both organic and mineral fertilisers by at least 50% by 2030, while ensuring no deterioration in soil fertility [10].
So here is the main question: how do we manage nitrogen responsibly to feed the growing population while sustaining the planet? The answer lies in soil bacteria. Only certain microbes (bacteria) have the ability to convert atmospheric nitrogen into a biochemically usable form of nitrogen through a process called Biological Nitrogen Fixation (BNF). This process involves a complex chemical language between the legume plant and soil bacteria called rhizobia. To successfully establish an interaction, legume plants and bacteria produce specific chemical signals that, if understood on both sides, lead them to recognise each other as “partners”. When this signal match happens, legumes allow rhizobia to enter their root cells, which induces the formation of specialised structures called nodules as a home for these bacteria [11]. Once rhizobia are inside, these bacteria switch off most of their genes and rely on the legume plant to provide them with the nutrients they need to survive in the form of carbohydrates. In exchange, these bacteria, now “symbiotic cells”, become “nitrogen factories” as they are able to process atmospheric nitrogen (N2, the compound with the strong triple bond difficult to break) during a reduction process (see Figure 4). In that way, they are able to biologically transform N2 into ammonia. If this sounds familiar to you, you are not wrong! This is the same process that results from the Haber-Bosch reaction [12] but, instead of requiring iron catalysts, high temperatures, and high pressures… Everything happens naturally due to a biological process called mutualism or symbiosis: they are “biological fertilisers”!
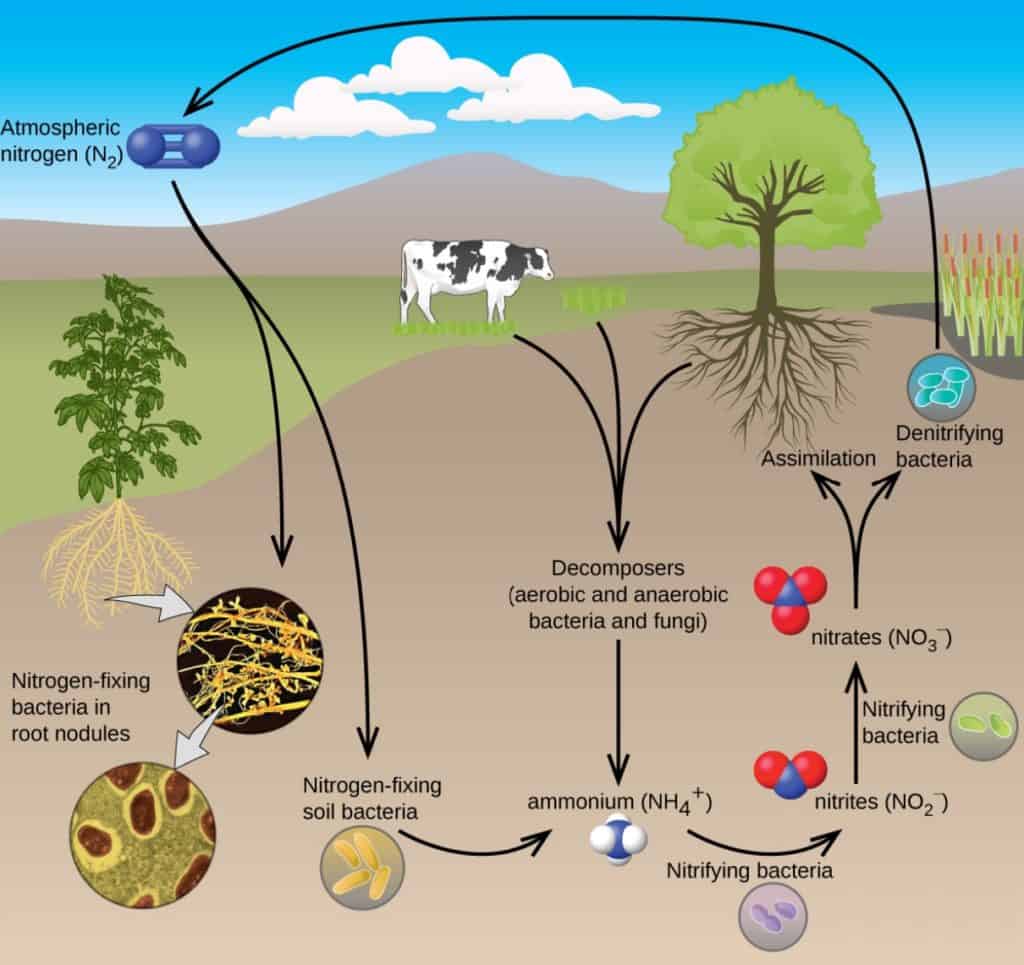
This biological process primarily occurs when the soil bacteria associate with legume plants, making legumes essential in crop rotation programs worldwide. In agriculture, this symbiosis reduces the reliance on chemical fertilisers, and thus taking advantage of soil resources whilst maximising crop production and hence reducing the costs, ecological impact, and fossil fuel consumption attendant on large-scale application of fertilisers [13]. However, there are still many challenges to use these bioinoculants as a routine agricultural practice for every crop. First, although wheat, rice, maize, and other cereals are the major crops worldwide, they are unable to interact with rhizobia, and hence have to rely on chemical fertilisers. In addition, this natural relationship between bacteria and legumes is so intricate that a particular bacterium will only associate with a specific number of plants — they are very selective! What’s more, commercial inoculants designed based on specific bacteria from a particular soil might not work when applied to a different one, as they often fail to compete against their native soil bacteria, resulting in low yields.
Understanding the molecular processes responsible for the establishment of this symbiosis will lead to sustainable crop yields and a positive impact on the environment, being the current goal in this area of research engineering biological nitrogen fixation into cereal crops [14]. The relevance of this area of research relies on the environmental benefit of controlling microbial nitrogen fixation, overcoming the use of chemically synthesised fertilisers to soils for a sustainable crop production. Different approaches are followed at the moment:
- Engineering cereals to turn themselves into fertiliser factories by inserting the nitrogenase genes coding for the key protein complex responsible for biological nitrogen fixation.
- Engineering cereals so that they can mimic the chemical language and establish a symbiotic relationship with nitrogen-fixing bacteria.
- Engineering nitrogen fixation into existing microbes that already colonise cereal plants to make them release ammonia in exchange for carbohydrates made by their host plant.
As Fritz Haber said in his Nobel Prize speech in 1918, referring to the Haber-Bosch process: “It may be that this solution is not the final one. Nitrogen bacteria teach us that Nature, with her sophisticated forms of chemistry of living matter, still understands and utilises methods which we do not yet know how to imitate”.
By Carmen Sánchez Cañizares (@carmen_agro). Postdoctoral researcher at University of Oxford, UK.
More information:
- World Population (2021). Source: Nations, U., Department of Economic and Social Affairs. Available online here.
- EU consumption of crops for biofuels could feed around 150 million people (2022). Source: CONCITO. Available online here.
- Food Matters: Towards a strategy for the 21st Century (2008). Source: Cabinet Office Strategy Unit. Available online here.
- Highfield R. (2011).
- Clara Immerwahr and the moral responsibility of science (2022). Source: SRUK/CERU Blog, post written by Carmen Sánchez Cañizares. Available online here.
- Smil V. (1999).
- Fryzuk MD. (2004).
- Feast or Famine: Fertilizer Use Worldwide, a Story of Deficiency and Excess (2013). Source: National Geographic. The original post cannot be found, although a link to the map can be found on Maps on the Web here.
- Fertilizer prices expected to remain higher for longer. Source: World Bank Blogs, post written by John Baffes and Wee Chian Koh. Available online here.
- The European Grean Deal, a communication from the European Commission. Source: EUR-Lex. Document available online here.
- Oldroyd G. et al. (2011).
- Dixon R. and Kahn D. (2004).
- Griesmann M. et al. (2018).
- Stokstad E. (2016).
Additional reading: You may want to consult Olaya Muñoz Azcárate’s post published in the SRUK/CERU Blog in 2016 entitled “Pulses: the important ecological value of staple food” as it complements the content of this post. Available online here.