When we talk about the intestinal barrier, we usually think about mucus and its epithelial cells. While this is indeed true (we already talked about this in the SRUK/CERU Blow some time ago), there is a lesser-known yet crucial component: the intestinal glycocalyx. Even though the glycocalyx is not a new cellular component (its discovery dates back to 1969 [1]), it has only been quite recently that we have discovered its function in vitro (we also wrote another post in this blog to discuss this).
This protective layer, which mainly consists of proteins called membrane mucins, covers the intestinal cells and acts as a first line of defense against microorganisms and toxins in vitro. In fact, our research group discovered that MUC17 is one of the main components of the intestinal glycocalyx and starts to develop during infancy, just when microbiota colonises our gastrointestinal tract.
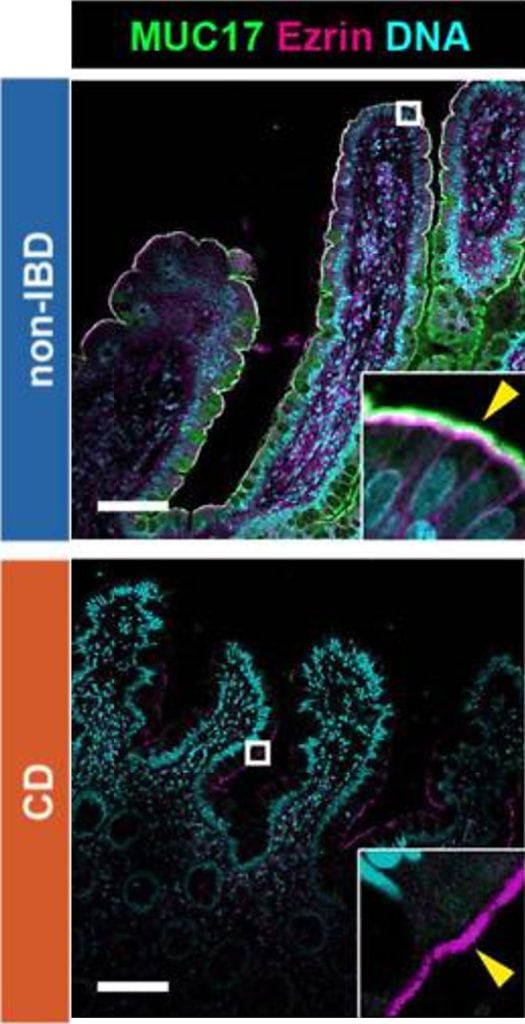
Image based on Fig. 1 inLayunta et al. (2024).
Nevertheless… What happens if this line of defense weakens? Our latest findings, published in the Journal of Clinical Investigation Insight [2], show that when there is less MUC17, the intestine becomes more exposed to infections. As if that was not striking enough, we also observed that reduced levels of MUC17 could be linked to Crohn’s disease, a chronic inflammatory bowel pathology. In this new study, we found that patients with Crohn’s disease have reduced levels of MUC17 as shown in Figure 1.
Under this scenario, bacteria and intestinal epithelial cells tend to be closer, which could be key to understand how the disease evolves. Crohn’s disease is an inflammatory disorder that can affect any part of the digestive tract, although it is usually found in the small intestine. Its origin is still unclear, but it is known that certain disruptions in the intestinal barrier allow bacteria to enter, which triggers abnormal immune responses.
To further explore the role of MUC17, we developed a conditional knockout mouse model to study the gene responsible for this glycocalyx component: Muc17 gene. We genetically modified this type of mice to ‘deactivate’ this gene in intestinal epithelial cells. These mice cannot produce MUC17, which makes them resemble patients suffering from Crohn’s disease, and thus allows us to further study the role of this gene.
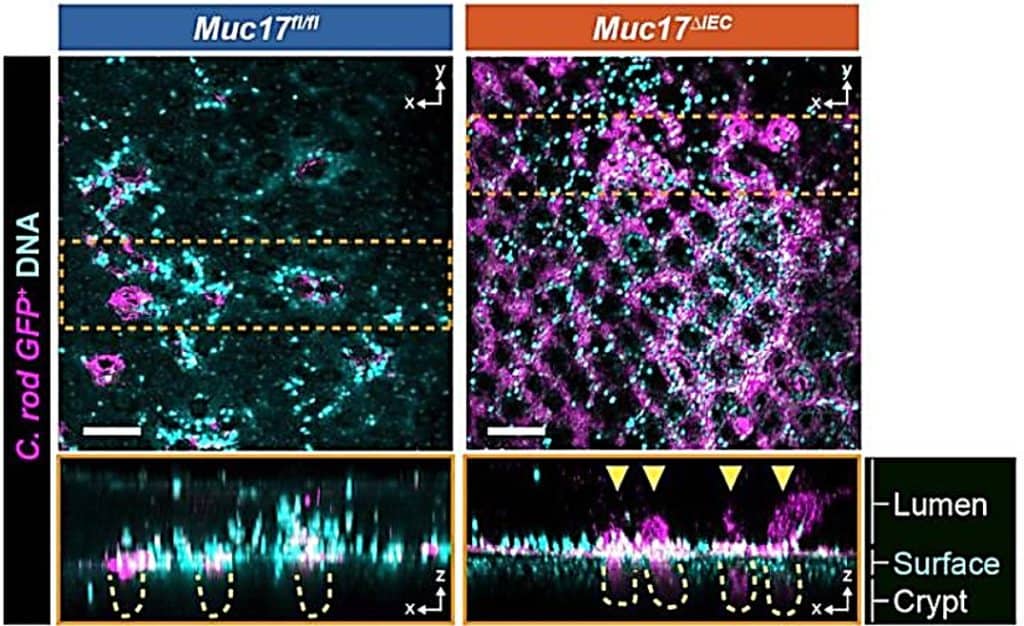
Our results were clear: these mice were more vulnerable to atypical intestinal infections (see Figure 2), showed disrupted epithelial homeostasis, and even had an abnormal microbiota (see Figure 3). Consequently, the absence of MUC17 seems to foster an environment conducive to chronic inflammation, as well as it is observed in Crohn’s disease.
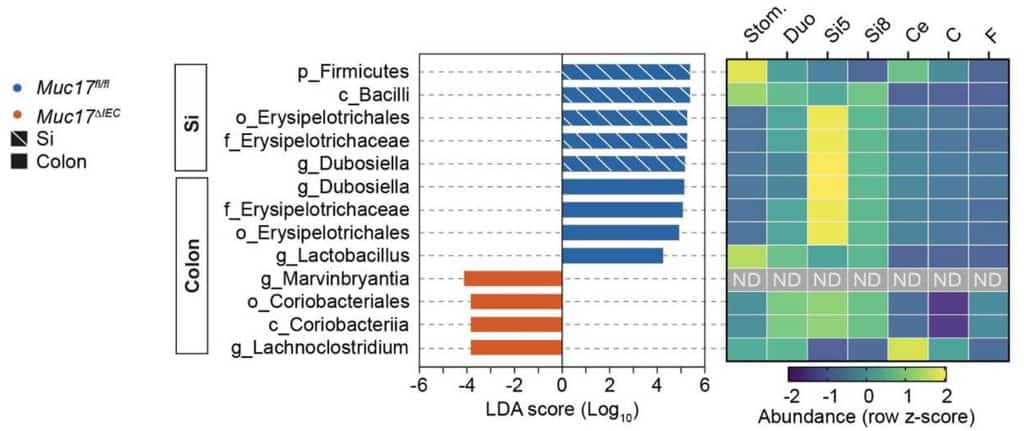
This discovery marks a turning point: if the absence of MUC17 can alter both microbiota and intestinal homeostasis, could other environmental factors—such as diet or stress—worsen this issue? The effects of external factors may be key to understand how some people develop intestinal diseases, even when there is no genetic predisposition. Now, the main question: how can we strengthen this intestinal barrier to prevent gastrointestinal disorders? If reduced MUC17 contributes to intestinal barrier disruption, one possible strategy could focus on stimulating its production or stability within the barrier. In this context, IL-22 (an interleukin that helps repair damaged tissue and strengthens natural barriers such as the intestinal lining) may be an interesting molecule for future research given its role in how the MUC17 glycocalyx develops during infancy.
Currently, many questions such as the following still remain unanswered: what other factors could promote the formation and stability of the intestinal glycocalyx? Could MUC17 become a new therapeutic target for Crohn’s disease and other gastrointestinal disorders? If MUC17 is key target, could we train our microbiota to help maintain our intestinal barrier in an optimal state? Perhaps one day intestinal diseases may be prevented by closely monitoring diet and microbial colonisation of the gut during infancy, which implies following preventive measures from the very first moments of life.
When one door closes in research, many more tend to open. Rarely does one live long enough to fully close a research topic, and that is what keeps us going! Until your last breath, you can always be excited about discovering something new! As long as the glycocalyx keeps revealing secrets about how the gut system works, we shall keep playing detective. See you in the next chapter of this mystery!
By Elena Layunta Hernández (@ELayunta). Assistant Professor at the Department of Anatomy, Embryology, and Animal Genetics | Faculty of Veterinary | IUI Mixto Agroalimentario of Aragón (IA2) | University of Zaragoza.
More information:
- Ito S (1969).
- Layunta et al. (2024).
- Layunta et al. (2021).
- Mucin Biology Groups. Source: University of Gothenburg. Available via this link.
- Membrane Mucin Groups. Source: Pelaseyed Lab. Available via this link.